Regulatory affairs (RA) is a profession in the life science industry that involves checking whether a business is following official rules or laws regarding the safety and efficacy of products. Regulatory affairs professionals supervise the developing, testing, manufacturing, and marketing of products in areas such as pharmaceuticals, medical devices, cosmetics, pesticides, and complementary medicines. They also work with regulatory agencies and personnel on specific issues that affect their business and advise their companies on the regulatory aspects and climate that would affect proposed activities.
This blog post will discuss regulatory affairs in detail, what regulatory affairs professionals do, and how PSC Biotech can help you achieve your regulatory goals.
RA is a specialized field within the life sciences industry that deals with the regulatory approval and compliance processes for new and existing products like pharmaceuticals, medical devices, biologics, and other healthcare products. These products are primarily designed to have therapeutic value and help treat patients or other consumers. RA comes into view when these products must meet the stringent regulatory requirements set by government agencies like the FDA (Food and Drug Administration) in the United States or the EMA (European Medicines Agency) in Europe.
Key aspects of its significance include:
- Product Approval: Obtaining approvals for new drugs, medical devices, and therapies from respective regional government regulatory agencies. This process involves extensive documentation indicating the design, research and development, non-clinical and clinical trials, studies and reports, and interactions with regulatory agencies to demonstrate the safety and efficacy of the product.
- Quality Assurance: Products must be manufactured, labeled, and tested in compliance with regulations to maintain consistent quality and safety.
- Compliance: Companies must navigate complex and evolving regulations, ensuring ongoing compliance with changing requirements.
- Risk Management: All the risks associated with regulatory issues must be managed and assessed efficiently, guiding companies in minimizing potential legal and financial risks.
- Market Access: Regulatory approvals are often prerequisites for market access. Approvals are granted by government agencies via drug submissions/ dossiers, i.e., documents that indicate all the quality, safety, and efficacy data.
- Patient Safety: Ensuring that products are safe and effective is paramount to protecting patients’ well-being.
Overall, RA is vital for the life sciences industry to bring innovative and safe healthcare products to the market while ensuring they meet rigorous regulatory standards. Failure to comply with regulations can result in product recalls, legal consequences, patient adverse events, and damage to a company’s reputation.
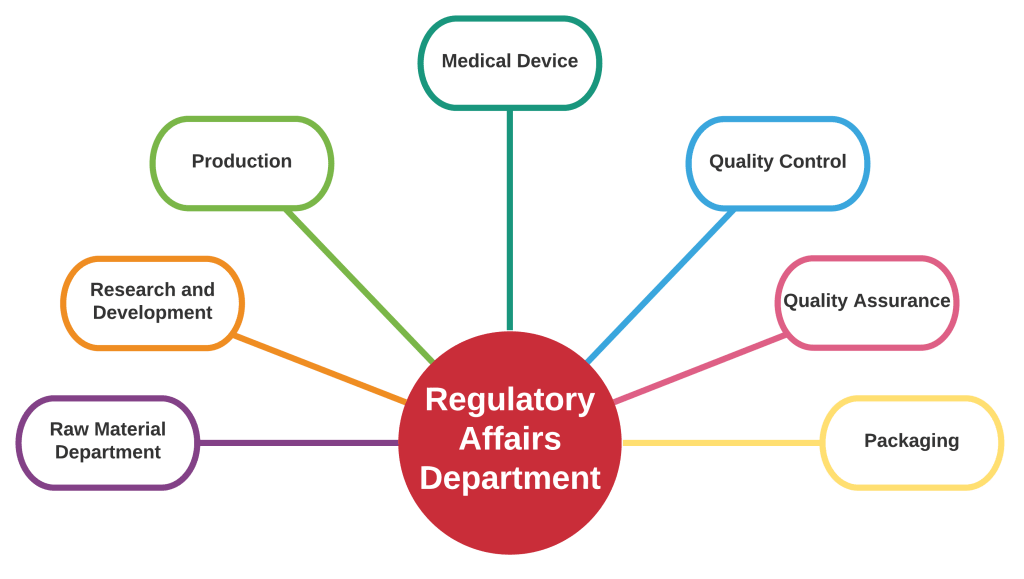
Role of Regulatory affairs and how it is connected with all departments. Source: Pharma Advisor
What do Regulatory Affairs professionals do?
RA professionals play a crucial role in the life sciences industry by managing the complex regulatory processes that govern the development, approval, and marketing of pharmaceuticals, medical devices, biologics, and other healthcare products. Their responsibilities encompass a wide range of tasks, including:
- Regulatory Submissions: RA professionals prepare and submit applications, dossiers, and documents to regulatory agencies seeking approval for new products or changes to existing ones. This involves compiling data on safety, efficacy, and quality.
- Compliance: RA professionals ensure that their organizations adhere to all relevant regulations, standards, and guidelines. They monitor changes in regulations and advise on necessary adjustments to maintain compliance.
- Clinical Trials: They may oversee the planning and execution of clinical trials, ensuring they meet regulatory requirements and ethical standards. They collaborate with clinical and research teams to design trials that generate acceptable data for approval.
- Labeling and Packaging: RA professionals provide input on product labeling and packaging to ensure it accurately reflects product information and complies with regulatory requirements.
- Quality Assurance: Regulatory professionals work with quality assurance teams to establish and maintain robust quality management systems (QMS). This includes managing documentation, audits, and corrective and preventive actions (CAPAs).
- Regulatory Strategy: They develop and implement regulatory strategies for bringing products to market efficiently and effectively. This includes considering global regulatory requirements and market access strategies.
- Communication with Regulatory Agencies: They represent their organizations in communications with regulatory authorities, addressing inquiries, providing additional data, and participating in regulatory meetings and negotiations.
- Post-Market Surveillance: After a product is approved and is on the market, they monitor its performance, including adverse event reporting, to ensure ongoing safety and efficacy.
- Global Regulatory Affairs: They navigate various regulatory systems and requirements in international markets, ensuring that products can be marketed globally.
- Risk Management: They assess and manage risks related to regulatory compliance, keeping the organization informed about potential regulatory challenges and opportunities.
- Training and Education: RA professionals often provide training to internal teams to ensure that employees understand and follow regulatory processes and requirements.
- Ethical and Legal Considerations: They uphold ethical and legal standards in all interactions with regulatory agencies, healthcare professionals, and the public.
RA professionals ensure that healthcare products meet regulatory standards, are safe and effective, and can be brought to market in compliance with all applicable laws and regulations. Their work is essential to the success and integrity of the life sciences industry.
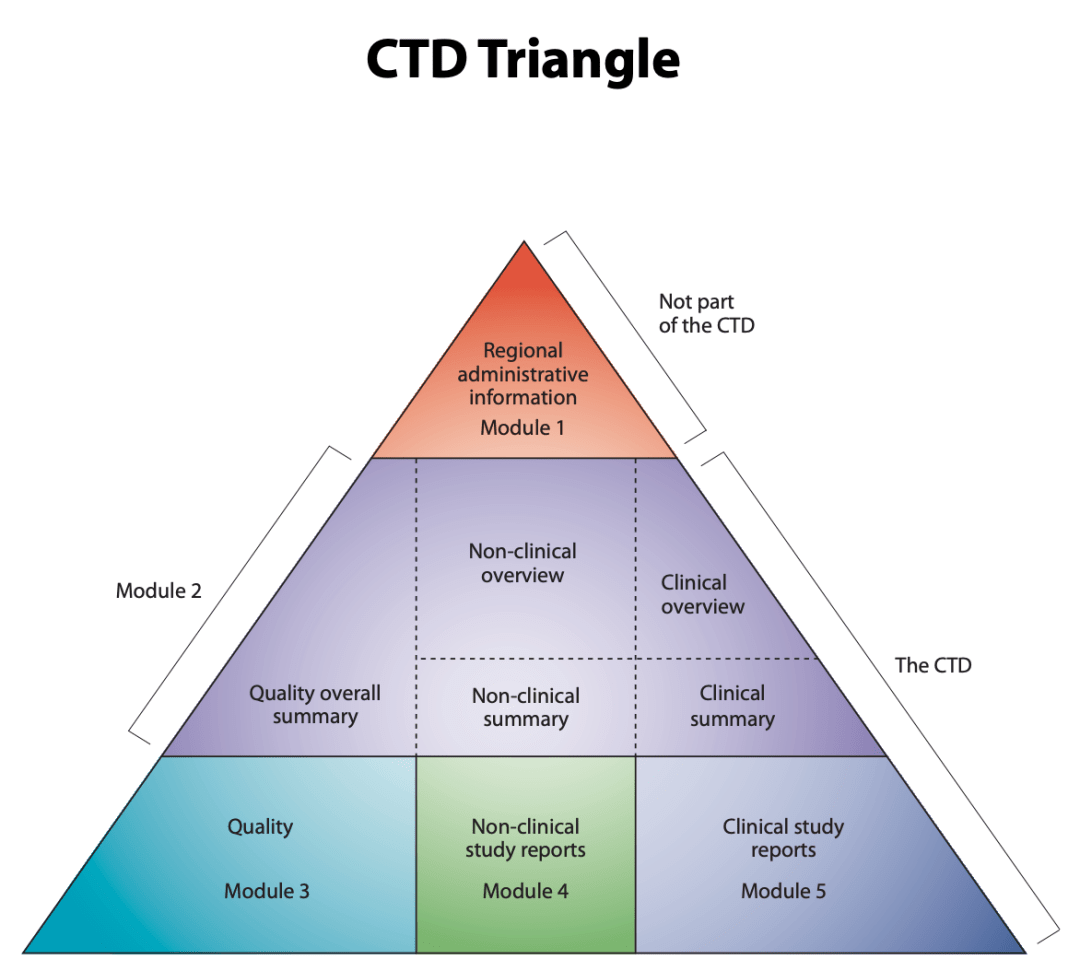
The CTD triangle. The Common Technical Document is organized into five modules. Module 1 is region specific and modules 2, 3, 4 and 5 are intended to be common for all regions. Source: ICH Website
How can PSC Biotech help you in accomplishing your Regulatory goals?
PSC’s RA team has vast knowledge and experience in submitting global regulatory documents. We support all regulated product submissions for pharmaceuticals, medical devices, biologicals, food, beverages, cosmetic products, and tissue products.
We can support pre-market and post-market activities for global life science submissions. We have worked on various regulatory projects involving new product registrations in international markets, development and analysis of regulatory strategies, submission documents, and guidance on regulations for each product type.
Our Specialties include:
Regulatory Strategy
- Estimate regulatory risks to your product.
- Review labelling requirements based on the product and the specific markets.
- Review pre-clinical, product process,, and manufacturing data.
- Review non-clinical and clinical study documentation (Phase 0, Phase I, Phase II, Phase III, Phase IV clinical trial studies)
- Submission scheduling and approval metrics.
Review and maintain Project documentation and Records:
- Author or review product quality-related documentation, chemistry, manufacturing, and controls (CMC) related documentation, & remediation projects
- Review licensing applications, dossier templates
- Assess or evaluate pre-approval and post-approval documentation, clinical trial forms, post-approval documentation, BA/BE studies, and Common Technical Document, also known as CTD modules.
- Response to Agency questions, requests for Orphan drugs and fast-track designations, protocol assistance
Generate and Manage Submission files and Review Author submission files
- Author CTD Modules and eCTD Modules, review or manage submissions and dossiers such as NDA, BLA, ANDA, DMF, BMF, EUA, or any other new product submissions.
- Author, review, or manage medical device submissions such as 510(k), PMA, IDE, MDR, & labeling, and brand naming for all class devices (I, II, III)
- Develop or assess applications for licensing, pre-clinical, clinical trial studies, and other tests.
- Manage establishment registration, quality system regulation, and medical device listings.
- Guide changing regulations such as 21 CFR, ISO, EU GMP, ICH Guidelines or any other market regulations that can impact your product or processes.
- Manage all your global documents and management systems to ensure efficiency for all your submissions.
Risk-Benefit Analysis and Risk Assessments for Regulatory Compliance:
- Perform appropriate Gap and risk analysis to help companies analyze risk mitigation actions.
- Develop risk assessment and management programs for your product as part of pre-and post–approval submission processes.
- Perform mock audits to assess quality and regulatory compliance.
Develop Internal Training programs and provide Regulatory support to existing teams.
PSC Biotech also has the expertise to support other processes that can impact regulatory operations, such as Computer System Validation (CSV), Computer System Assurance (CSA), Technical Writing, Audits, Commissioning, Qualification and Validation (CQV), and project management activities.
See Also
PSC Software Solutions
Hire Consultants
PSC Biotech Services provide fast, budget-friendly, high-level expertise.
Follow Us
Share, Engage, Stay Current
Quality assurance (QA) is a process that helps a business ensure its products or services meet the quality standards set by the company or its regulatory requirements. QA is involved in all stages of a product’s development, from raw material delivery through…
The BIO International Convention is the premier event for the global biotechnology industry, where you can network with thousands of peers, leaders, and partners and discover the latest innovations and opportunities in the life science sector. This year, the…
Metrology is critical to any life science manufacturing process in a highly technical and regulated industry. At PSC Biotech, we offer a range of metrology services to help our clients maintain equipment and systems compliance requirements and ensure their…
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://biotech.com/2023/09/25/your-compass-for-navigating-biopharma-regulations/