The past five years have seen mainstream adoption of molecular and lateral flow point-of-care diagnostics. To combat the challenges caused by the pandemic, thousands of diagnostic products entered the market eventually paving the way for an accelerated development roadmap.
Nowadays, entrepreneurs that want to develop lateral flow or molecular diagnostics can simply find a specialized development firm that uses ready-made systems and sophisticated off-the-shelf (OTS) tooling to develop and commercialize a product in record time.
Though these ready-made solutions exist for molecular and lateral flow assays, accelerated roadmaps do not yet exist for haematological and quantitative immuno-assays (e.g. CBC panel, electrochemical biosensors, ELISA, etc.). These immuno-assays typically rely on accurate quantification of non-NAAT biomarkers (e.g. proteins, cytokines, small molecules, etc.) whereas hematological assays rely on assessment, identification and/or quantification of individual cells to arrive at a clinical diagnosis. It is exactly these types of assays that will provide the most promising opportunities for future sample-to-answer products that generate faster information for better clinical decision making.
To help pave the way for an accelerated developmental roadmap for haematological and quantitative immuno-assays, our team designed a ready-made modular proof-of-concept system. This blog discusses how this system can help expedite and de-risk product development in ways similar to what is done for lateral flow and molecular assays.
Developing a point of care diagnostic is all about experimentation and gathering data.
At a high level, successful development of point-of-care diagnostics, requires taking an analytical assay from a tightly controlled environment (e.g. a central lab) and successfully and robustly performing it in a less tightly controlled environment (e.g. point-of-care). Outside the laboratory, the point-of-care product needs to control for the impact of increased variability. Otherwise, it can play havoc on robustness and analytical performance. Individual drivers of variability include workflow variations, user training, storage conditions, age of the consumable, temperature and humidity, reagent lot-to-lot variations, the quality of specimen, etc.
During product design and development, the impact of individual drivers needs to be assessed. This can only be done through hands-on experimentation. The data from these experiments then feeds into design decisions. This is where developers find themselves in a bit of a conundrum because ideally one would start testing immediately, however; how could a developer start testing without having a proof-of-concept system in hand? And how to design and build an applicable proof-of-concept system without a proper understanding of requirements to control for variability in results?
How can a ready-made modular proof-of-concept system speed up development?
Leveraging off-the-shelf (OTS) components helps address part of this challenge. Especially parameters like “reagent lot-to-lot variability” or “variability due to specimen quality” can be investigated using traditional laboratory tests and OTS components. However, variability resulting from workflow variations (e.g. “how reagents are mixed” or “how long the reagents are incubated”, etc.) can often only be addressed and controlled by automation of the assay. Automation of an assay is typically accomplished by some form of controlled liquid handling inside a single-use consumable. The consumables handle the sample and the flow of wet and dry reagents while a multi-use instrument is used for data analysis.
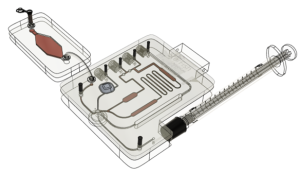
Creating a custom proof-of-concept system that automates the workflow can take a long time. This is why, lateral flow assays and molecular assays are often ported onto pre-existing systems to expedite development. Quantitative immuno-assays and haematological assays frequently need more customization than lateral flow and molecular assays which is why limited ready-made solutions exist and why we have designed a ready-made modular proof-of-concept system (Figure 1). By using this modular system, we can expedite testing with tooling that has already been shown to work without extensive modifications to the consumable and instrument.
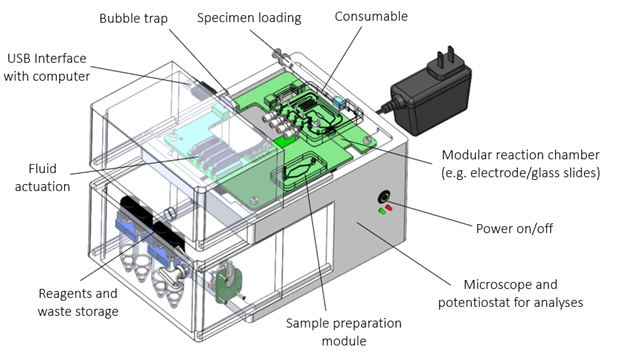
Figure 1. Modular Proof-of-Concept system as the first prototype for quantitative immuno-assays and haematological diagnostics. Based on assay requirements, modules can be included or excluded without extensive customization.
How does this speed up development in a real-world example? Let’s say that a client plans to develop a point-of-care diagnostic that can quantify a biomarker in blood. We like to start experimentation as soon as possible to understand variability and demonstrate technical feasibility of the product concept. This requires creating a proof-of-concept system that we can use in experiments. However, we would also like to postpone expensive and time-consuming custom product design until technical feasibility has been demonstrated. Using our modular system, we can rapidly port the assay onto a customizable microfluidic cartridge that controls fluid handling while using an accompanying ready-made proof-of-concept instrument to analyze the assay.
This effectively means that we immediately automate the workflow and start testing right away as opposed to starting with expensive and time-consuming custom design work. Of course, leveraging our ready-made modular proof-of-concept system does not mean that the product is in its final form factor. But this approach helps clients achieve technical feasibility in the most cost-effective and time-sensitive manner. Only after technical feasibility has been demonstrated, do we embark on design work to arrive at a custom diagnostic product in a more final configuration.
What exactly can the modular system do?
The modular system consists of a consumable and an instrument. To accommodate most immuno- and haematological assays, the modular consumable can control up to 4 dry and/or wet reagents (see Figure 2). These reagents can be mixed, incubated, reconstituted, heated/cooled, and combined with automated sample handling. If needed, the specimen can undergo preparation in a separate module (e.g. lysis, purification, filtration, etc.) to provide maximum flexibility.

Figure 2. Ready-made modular consumable that can accept a specimen, automate fluid handling and can be tailored to run a variety of different assays for early feasibility testing.
Bubbles can be removed from the fluidic channels by means of a bubble trap. Each fluidic channel can be opened or closed allowing for flexibility without the need to re-design the cartridge. A separate modular single-use reaction chamber is part of the cartridge. This reaction chamber can slide into the consumable housing and can include a variety of modalities (e.g. electrochemical biosensor, ELISA, optical chambers to assess individual cells, etc.).
The entire cartridge fits into a proof-of-concept instrument that actuates fluids and performs electrical or optical analysis. The system communicates data to a separate computer running OTS software for analysis. Customization of software analytics and/or algorithm development can be applied as required. Using this modular system, we can start testing almost immediately while controlling the workflow and providing the ability to optimize individual components (e.g. the electrochemical biosensor, the sample prep module and/or fine-tuning of data analysis, etc.).
Conclusion
The modular proof-of-concept diagnostic platform aids smart, time-sensitive and cost-effective development while allowing for customization after most technical risks have been retired. Modular tooling is incredibly helpful in accelerating development programs and retiring technical risk early. The approach outlined in this blog prioritizes early technical de-risking without over-spending while still arriving at custom and well-designed market-ready diagnostics that meet a defined market need. It will be exciting to see more point-of care diagnostic products going to market using this approach.
Image: StarFish Medical
Joris van der Heijden is Bio Services Program Manager at StarFish Medical. Previously he was Director of R&D at Spartan Bioscience where he led the development of a point-of-care COVID-19 diagnostic test. Joris received his PhD in infectious diseases from UBC.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://starfishmedical.com/blog/modular-rapid-prototyping/