| Apr 02, 2024 |
|
|
|
(Nanowerk Spotlight) Microfluidics, the technology of precisely controlling fluids at the submillimeter scale, has long held the promise of revolutionizing biological research and medical diagnostics. By miniaturizing assays into tiny water-in-oil droplets, these platforms can analyze individual cells with unprecedented speed and efficiency while drastically reducing reagent costs. Each picoliter-scale droplet serves as an isolated microreactor, allowing high-throughput studies of cellular behavior and enabling diverse applications from drug screening to rare cell analysis.
|
|
However, unlocking the full potential of droplet microfluidics has been hindered by the challenge of rapidly and comprehensively analyzing the contents of these minuscule compartments. Conventional approaches rely on complex and expensive microscopy setups, utilizing high-speed cameras to image each droplet as it flows through the device. The technical complexity and steep costs associated with such systems have limited the widespread adoption of droplet-based techniques in research and clinical settings.
|
|
Now, a multidisciplinary team of scientists has developed OptiDrop, an innovative device that ingeniously overcomes these limitations by integrating optical fibers directly within a microfluidic chip. This novel approach enables the detection of multiple optical parameters from individual droplets and their contents without relying on a microscope or camera. The result, reported in Microsystems & Nanoengineering (“Multiplexed fluorescence and scatter detection with single cell resolution using on-chip fiber optics for droplet microfluidic applications”), is a miniaturized, affordable, and user-friendly platform that brings the power of multiparameter single-cell analysis to a benchtop format.
|
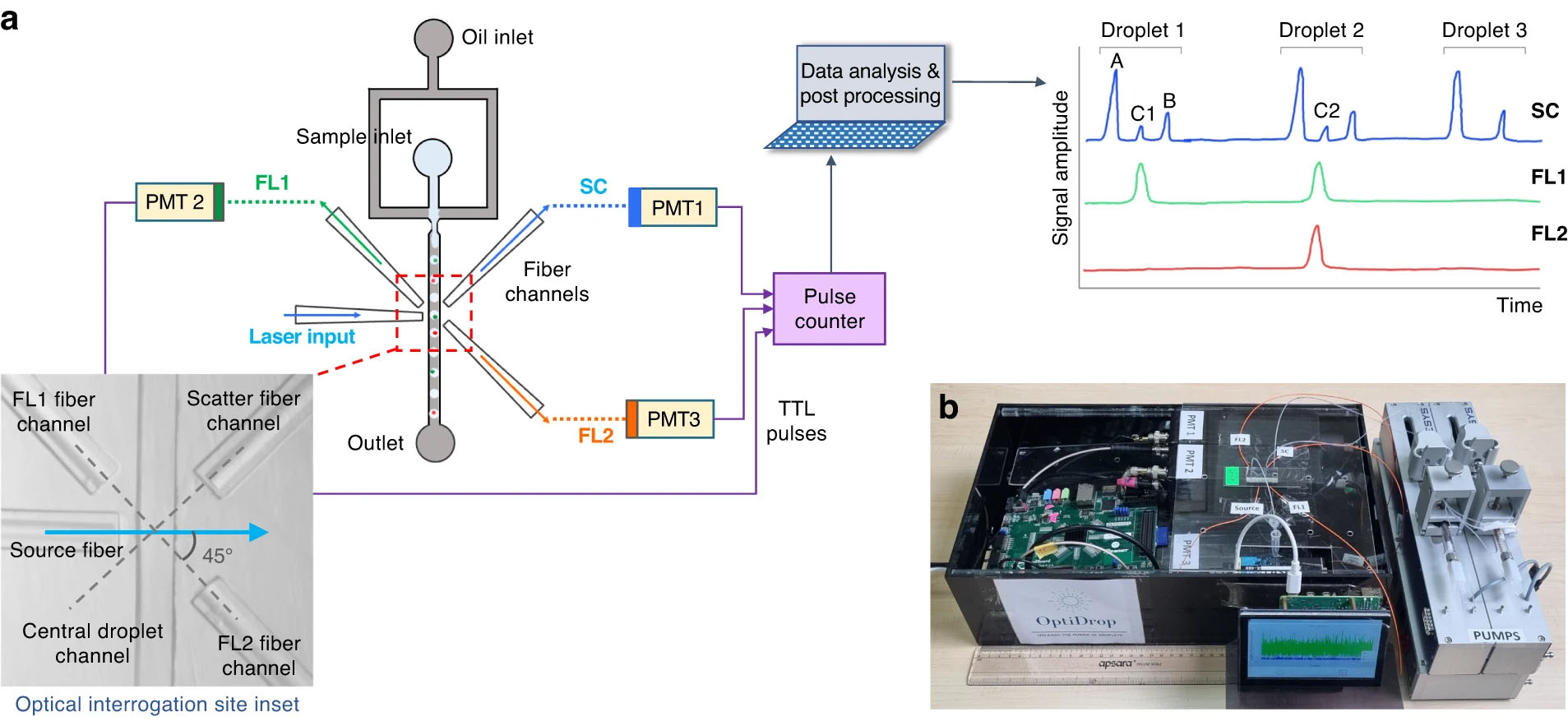 |
| a The OptiDrop platform comprises a microfluidic chip with an oil and water inlet for the formation of droplets at the flow-focusing junction. The aqueous phase is encapsulated within droplets and subsequently flows through the optical interrogation site (inset) flanked by a set of optical fiber grooves arranged around the central channel. The grooves on the chip are used to house the optical fibers at set angular positions, allowing for effective droplet illumination with the incident laser light and collection of scattered light and fluorescence signals as the droplet passes through the light beam. The optical fiber output is coupled into a PMT for detection. TTL pulse counts from each PMT are integrated by an FPGA chip with a pulse counter and plotted as raw signal intensity peak data. The raw data can be further analyzed to identify or measure fluorescence intensities from the cells of interest. b Benchtop assembled unit with a foot ruler for scale along with a live data stream viewing screen and syringe pumps. (Image: Microsystems & Nanoengineering, CC BY 4.0)
|
|
The core innovation of OptiDrop lies in the strategic positioning of optical fibers around the microfluidic channel. As each droplet passes through the interrogation point, a laser illuminates it, generating scatter and fluorescence signals. These optical signals are efficiently collected by fibers placed at 45° angles and then routed to separate detectors. Custom-built electronics digitize the signals in real-time, enabling instantaneous visualization and analysis of the optical profile of each droplet.
|
|
To validate the performance of OptiDrop, the researchers first characterized its sensitivity and dynamic range using standardized fluorescent dyes. Impressively, the device could detect dye concentrations as low as 1 nanoMolar, rivaling the limits of detection achieved by conventional instruments while requiring only a fraction of the sample volume. Moreover, the fluorescence intensity measured by OptiDrop scaled linearly across a wide range of concentrations, ensuring accurate and reliable quantification.
|
|
The researchers then demonstrated OptiDrop’s capabilities for particle analysis using microbeads of varying sizes and fluorescence intensities as cell mimics. The platform readily distinguished between beads based on both their physical dimensions and optical properties. Notably, even in a heterogeneous mixture of dimly and brightly fluorescent beads, OptiDrop accurately identified and enumerated each subpopulation. This highlights the system’s robustness in analyzing complex biological samples containing diverse cell types.
|
|
To showcase the platform’s utility for a biologically relevant application, the team conducted a live-cell assay investigating the surface expression of major histocompatibility complex (MHC) proteins on immune cells. MHC molecules are crucial regulators of immune responses against pathogens and abnormal cells, making their expression levels valuable biomarkers. By leveraging fluorescently labeled antibodies, OptiDrop allowed the simultaneous detection of both MHC class I and II proteins on individual cells encapsulated within droplets. Stimulation of the cells with interferon-gamma, an immunostimulatory cytokine, elicited the anticipated upregulation of MHC expression, which was sensitively quantified by the OptiDrop platform.
|
|
The development of OptiDrop marks a significant stride towards democratizing cutting-edge single-cell analysis capabilities. By overcoming the cost and complexity barriers that have hindered the adoption of droplet microfluidics, this innovative platform brings powerful cellular interrogation tools within reach of a wider range of researchers and clinicians. Its affordability, user-friendliness, and robustness make it well-suited for routine deployment in biomedical research laboratories and point-of-care diagnostic settings.
|
|
The potential applications of OptiDrop are vast and far-reaching. In the realm of disease monitoring, it could enable ultrasensitive detection of rare biomarkers, facilitating non-invasive early diagnosis and treatment response assessment. Integration with single-cell sequencing workflows may allow direct correlation of visual phenotypes with gene expression profiles, offering a more comprehensive understanding of cellular states. Furthermore, the platform’s closed design and ability to rapidly process clinical specimens make it particularly attractive for infectious disease testing and other time-sensitive diagnostic applications.
|
|
While OptiDrop represents a significant advancement, there is still room for further optimization and expansion of its capabilities. Increasing the throughput by enabling faster droplet flow rates and generating smaller droplets could enhance its efficiency and scalability. Integrating active sorting functionalities would empower users to isolate specific subpopulations of interest based on their optical signatures, enabling downstream molecular analyses. Moreover, the development of standardized cartridge designs and intuitive software interfaces will be crucial for streamlining its operation and data interpretation.
|
|
As our understanding of biological systems becomes increasingly nuanced, tools like OptiDrop that seamlessly integrate microfluidics, optics, and electronics will be indispensable for unraveling their intricacies. By providing a accessible and versatile platform for high-throughput single-cell analysis, OptiDrop equips researchers and clinicians with a powerful tool to ask new questions, challenge existing paradigms, and translate groundbreaking discoveries into meaningful real-world impacts. As the technology continues to evolve and mature, it holds immense promise for revolutionizing our approach to biological inquiry and precision medicine.
|
|

By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nanowerk.com/spotlight/spotid=64952.php





