Preparation of Mel-PLGA NPs
One-step nanoprecipitation-solvent volatilization method was used for the synthesis of Mel-PLGA NPs26. Twenty mg of PLGA (P2191, lactide:glycolide (50:50), mol wt 30,000–60,000, Sigma Aldrich, USA) was dissolved in acetone (one ml); and two mg of Mel (73-31-4, mol wt 232.28, Sigma Aldrich, USA) were added followed by half an hour of centrifugation to form the organic phase [2% (w/v)]. The formed organic phase was injected into distilled H2O with continuous stirring for half an hour; followed by evaporation of acetone (at 37°C under vacuum).
Characterization of Mel-PLGA NPs
The produced Mel-PLGA NPs was obtained by freeze drying and stored at 4°C. The shape of prepared nanoparticles was observed by transmission electron microscope (TEM). Malvern.
Zetasizer device was used to determine the size and zeta potential of nanoparticles.
Encapsulation efficiency (EE%) and drug loading (DL%) of Mel-PLGA NPs26
Mel amount in Mel-PLGA NPs was determined by high performance liquid chromatography (HPLC). Where, Mel-PLGA NPs were dissolved in acetone followed by ultrasound to release the encapsulated Mel. The solution was centrifuged at 3000 rpm for twenty minutes to precipitate PLGA. The Mel dissolved in the supernatant represented the encapsulated mass in NPs. The Mel release from Mel-PLGA NPs was determined by diluting one ml of NPs with nine ml phosphate buffered saline (PBS, pH 7.4); followed by incubation at 37°C on a shaker. At 0, 20, 40, 60, 80 and 100 h, 300 µl of the solution were removed (replaced with the same volume with PBS) and centrifuged at 3000 rpm for 30 min. The EE% and DL% were calculated by the following equations:
$${text{EE}}% = left( {{text{mass}};{text{of}};{text{Mel}};{text{in}};{text{Mel}} – {text{PLGA}};{text{NPs}}/{text{total}};{text{mass}};{text{of}};{text{Mel}}} right) times {1}00.$$
$${text{DL}}% = left( {{text{mass}};{text{of}};{text{Mel}};{text{in}};{text{Mel}} – {text{PLGA }};{text{NPs}}/{text{total}};{text{mass}};{text{of}};{text{Mel}} – {text{PLGA}};{text{NPs}}} right) times {1}00.$$
In vitro effects of Mel-PLGA NPs
In vitro antioxidant effect of Mel-PLGA NPs 27
The antioxidant capacity of prepared Mel-PLGA NPs has been assessed from their free radical scavenging effects via 1, 1- diphenyl-2-picryl hydrazyl [DPPH (281,689, Sigma Aldrich, USA)]. Simply, different concentrations of NPs (from 3.9 to 1000 μg/ml) were mixed with one ml of DPPH/ethanol solution (0.1 mM), shaken, and allowed to stand for 30 min at 25°C. The absorbance was measured at 517 nm using ascorbic acid as the reference substance. DPPH scavenging activity% = [(A0–A1)/A0]× 100. Sample absorbance was A1, while control reaction absorbance was A0.
In vitro cytotoxicity effect of Mel-PLGA NPs
The safety of using Mel-PLGA NPs was examined, in vitro, before being used in vivo. Caco2 cells (Sigma Aldrich, USA) were cultured, at 37 °C in 5% CO2 and relative humidity of 95%, in Dulbecco’s modified Eagle medium (DMEM) supplemented with NaHCO3 (2.2 g/l), d-glucose (4.5 g/l), 1% non-essential amino acids, 10% fetal bovine serum, 100 IU/ml penicillin and 0.1 mg/ml streptomycin (all materials used in the culture process were purchased from Sigma Aldrich, USA). In vitro cytotoxicity assay were accomplished according to Alaa et al.28. 100 µl/well of 105 Caco2 cells in tissue culture plates were incubated at 37 °C for 24 h to allow for the development of cell monolayers. After medium decantation, a washing media was used to wash the monolayers. Graded concentrations of Mel-PLGA NPs were produced by combining NPs with RPMI medium. The produced NPs dilution was diluted to 0.1 ml, added to the wells, and then left to sit for another 24 h. The wells received 20 µl of MTT (3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide) at a concentration of 5 mg/ml. Plates were shaken for five min to ensure MTT mixing, then incubated for four h at 37°C with 5% CO2. To dissolve the developed formazan, 200 µl of dimethyl sulfoxide (DMSO) were applied to the plates. At 560 nm, the absorbance (which was directly linked to formazan) was measured.
In vitro coagulation effect of Mel-PLGA NPs29
The coagulation activity of prepared Mel-PLGA NPs was tested to predict their effect when administrated in vivo. The anticoagulant activity of Mel-PLGA NPs was assessed by measuring the clotting time in seconds at 37°C, with heparin serving as the control. According to the manufacturer’s recommendations, prothrombin time (PT) and partial thromboplastin time (PTT) reagents (pre-incubated at 37°C for 5 min) were utilised. Briefly, rat plasma (900 μl) and various Mel-PLGA NPs concentrations (100 μl) or heparin dissolved in saline were combined. The test was completed three times, and the clotting time was recorded.
In vitro anti-inflammatory (hemolysis inhibition) effect of Mel-PLGA NPs
The anti-inflammatory effect of Mel-PLGA NPs was determined by the hemolysis inhibition test according to Anosike et al.30. Fresh rat heparinized blood (5 ml) was centrifuged at 2500 rpm for 15 min; after that, the resultant pellet was dissolved with isotonic buffer (that was equivalent to the supernatant volume). Different doses of Mel-PLGA NPs (from 100 to 1000 μg/ml) were combined with 5 ml of distilled water to create a hypotonic solution. The same dosages of NPs were combined with an isotonic solution (5 ml); and indomethacin was employed as a control. NPs solutions and control received 0.1 ml of the produced erythrocyte suspension, which was then incubated for an hour at 37 °C before being centrifuged for three minutes at 1500 rpm. A spectrophotometer was used to quantify the released haemoglobin in the supernatant at 540 nm, and the percentage of hemolysis inhibition was estimated using the formula: hemolysis inhibition (%) = 1−[(ODb−ODa)/(ODc−ODa)]× 100. ODa stood for sample absorbance in an isotonic solution, ODb for sample absorbance in a hypotonic solution, and ODc for control absorbance in a hypotonic solution.
Animals and experimental design
Male Sprague Dawley rats, weighting 200 g and eight weeks age, were purchased from the animal house of National Organization for Drug Control and Research (Cairo, Egypt). All experimental procedures were carried out in accordance with the international guidelines for the care and use of laboratory animals and complied with the ARRIVE guidelines. Two doses of Mel-PLGA NPs (5 and 10 mg/kg) were examined in vivo to test the efficacy of NPs in treatment of CCL4-induced liver injury; and also, to find the required therapeutic dose. Moreover, two doses of free Mel (5 and 10 mg/kg) were used in the experimental subgroups and compared to the Mel-PLGA NPs administrated subgroups to proof the success of prepared Mel-PLGA NPs in reducing the amount of administrated Mel. Healthy control subgroups were designed as the subgroups with CCL4-induced liver injury to achieve a critical comparison and statistical analysis. Therefore, animals were divided into two groups, healthy (H) and CCL4-liver injured (I); each group was divided into five subgroups (five rats/subgroup):
-
Healthy (H) group:
H control GI: negative healthy control rats.
H Mel (5 mg/kg) GII: healthy control rats that received 5 mg/kg of Mel.
H Mel (10 mg/kg) GIII: healthy control rats that received 10 mg/kg of Mel.
H Mel-PLGA NPs (5 mg/kg) GIV: healthy control rats that received 5 mg/kg of Mel-PLGA NPs.
H Mel-PLGA NPs (10 mg/kg) GV: healthy control rats that received 10 mg/kg of Mel-PLGA NPs.
-
CCL4-liver injured (I) group:
I control untreated GI: untreated rats with CCL4-induced liver injury (positive control).
I Mel (5 mg/kg) GII: rats with CCL4-induced liver injury treated with 5 mg/kg of Mel.
I Mel (10 mg/kg) GIII: rats with CCL4-induced liver injury treated with 10 mg/kg of Mel.
I Mel-PLGA NPs (5 mg/kg) GIV: rats with CCL4-induced liver injury treated with 5 mg/kg of Mel-PLGA NPs.
I Mel-PLGA NPs (10 mg/kg) GV: rats with CCL4-induced liver injury treated with 10 mg/kg of Mel-PLGA NPs.
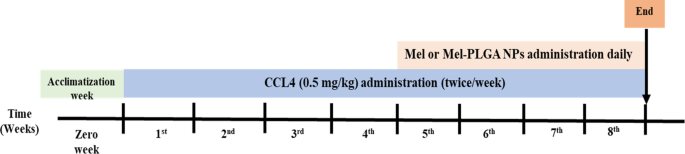
In addition to vehicle group (VG) that was composed of healthy rats that received 0.1 ml olive oil by intraperitoneal (i.p) injection twice/week throughout the experimental period. CCL4 was dissolved in olive oil and administrated at a dose of 0.5 mg/kg by i.p route twice/week for four successive weeks. After liver injury induction, Mel or Mel-PLGA NPs was administrated by i.p route daily for another four weeks (note: rats continued to receive CCL4 doses during treatment) (Fig. 1).
At the end of experiment, rats were terminally anesthetized with 50 mg/kg of sodium pentobarbital31. Blood samples were collected via cardiac puncture. After allowing the blood to clot at room temperature, serum was collected after centrifugation at 1500 rpm for 15 min and divided into aliquots to be kept at −20°C. Rats, from all experimental groups, were dissected for organ (liver) collection. Briefly, rat was placed on its back on the dissecting tray and its limbs were fixed by the aid of a tape. Rat’s skin was cut to expose the underlying muscles. The abdominal wall was peeled back and the liver was removed carefully. Liver specimens (one g), from all experimental groups, was homogenized using cold Tris–HCl buffer to prepare liver homogenate (10%).
Biochemical analysis in serum samples
Liver function parameters were measured in order to evaluate the hepatoprotective effect of Mel-PLGA NPs. The levels of aspartate aminotransferase (AST), alanine transaminase (ALT), albumin (ab234579, ab263883, ab108789, abcam, USA) and total bilirubin (MBS9389057, MyBiosource, USA) were measured by rat ELISA kits according to Farid et al.27.
Oxidative stress markers in liver tissue homogenates:
The antioxidant effect of prepared NPs was determined, in vivo, by measuring the lipid peroxidation marker malondialdehyde (MDA) and the antioxidant enzymes. MDA (MBS268427, MyBioSource, USA), GPx (MBS744364, MyBioSource, USA), SOD (ab285310, USA) and CAT (P04762, CUSABIO, USA) levels were measured by rat’s ELISA kits according to Farid et al.32 and Amr et al.33.
Cytokines levels in liver tissue homogenates
The anti-inflammatory effect of Mel-PLGA NPs was determined by measuring the level of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) and the anti-inflammatory cytokine (IL-10). Cytokine levels were measured in rats’ liver by IL-1β (MBS825017, MyBioSource, USA), TNF-α (ab100785, abcam, UK), IL-6 (P20607, CUSABIO, USA) and IL-10 (P29456, CUSABIO, USA) rat’s ELISA kits according to Farid et al.34.
Matrix metalloproteinases levels in liver tissue homogenates:
The Mel-PLGA NPs effect on liver tissue remodeling was evaluated by measuring MMP9 and TIMP1 by rat’s ELISA kits (MBS722532 and MBS2502910, respectively; MyBioSource, USA).
Flow cytometry technique
Flow cytometry technique was used to find the effect of prepared NPs on apoptosis and intracellular apoptotic proteins levels. Hepatocytes cell cultures were produced under sterilized conditions. The portal vein of rats under anaesthesia was perfused with collagenase buffer. The liver was dissected after perfusion, the cells were separated, suspended in William’s complete medium, filtered through a nylon filter (100 μm), and then cultivated. The level of apoptosis in liver cells was examined using the Annexin-V-FITC/PI apoptosis detection kit (ab14085, abcam, USA). Liver cells were permeabilized by saponin (pH 7.4); and the anti-apoptotic protein Bcl2 (11-6992-42) and pro-apoptotic proteins [Bax (MA5-14,003), p53 (ab90363), caspase 3 (C92-605) and 8 (ab32125)] were measured by flow cytometry.
Histopathological and immunohistochemical examination:
Liver sections were examined by ordinary hematoxylin and eosin staining method to assess the different histopathological changes between experimental groups. Immunohistochemical staining was used to evaluate the anti-inflammatory effect of Mel-PLGA NPs. The liver samples were dehydrated using increasing levels of alcohol: 70% alcohol for 1.5 h, 90% alcohol for 1.5 h, and absolute alcohol for 3 h. The liver was then cleared for 4 h in xylene. Following clearing, the liver specimens undergo the infiltration procedure, where they were impregnated with soft, pure paraffin via three distinct grades (each lasting one hour) at 56 °C. The specimens were then arranged in blocks and immersed in paraffin wax at 58 °C. For histological analysis, paraffin slices of 4 micron thickness were cut, stained with hematoxylin and eosin, mounted in dibutylphthalate polystyrene xylene, and then covered33,35. For immunohistochemical examination28, H2O2 (3%) [followed by a PBS wash and a 60-min blocking with bovine serum albumin (BSA, 5%)] was used to inhibit the endogenous peroxidase activity. Liver sections were washed in PBS, after, a 30-min incubation with the primary antibody [anti-nuclear factor-kappa beta (NF-кB) p65 antibody (ab86299, abcam, USA) or anti-C-Reactive Protein antibody (C1688, Sigma Aldrich, USA)]. The horseradish peroxidase (HRP)-rabbit anti-rat IgG secondary antibody (ab6734, abcam, USA) was applied to liver sections and incubated for 60 min. 3, 3-diaminobenzidine (DAB) was used for colour development, with the brown colour signifying a positive result. Liver sections were washed and then counterstained with hematoxylin (0.1%).
Statistical analysis
The data were expressed as mean ± SD and investigated by one way analysis of variance (ANOVA) using SPSS version 20.0 (SPSS Inc., Chicago, USA). Differences between means were examined by Tukey post hoc test. When P < 0.05, values were considered significant.
Ethics approval
All experimental procedure and animal maintenance were approved by the Institutional Animal Care and Use Committee.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-023-43546-4