F16BP microparticles are in phagocytosable range
To demonstrate the strength of such a strategy PFK15 (blocks−6-Phosphofructo-2-Kinase Fructose-2,6-Biphosphatase 3-PFKFB3) was chosen to block glycolysis, as it is one of the rate-limiting steps in glycolysis20. Moreover, Fructose-1,6-BiPhosphate (F16BP), which is generated by phosphofructokinase (PFK) that is a step downstream of PFKFB3 was chosen as the metabolite to generate phagocytosable particles21. F16BP-based microparticles were generated using calcium-phosphate chemistry. Dynamic light scattering demonstrated that the size of these particles was 2.3 ± 0.4 µm (Fig. 1b) and scanning electron micrographs showed that these particles had smooth spherical morphology (Fig. 1c). Using 1H NMR and EDX-mapping it was determined that the F16BP was incorporated within these microparticles (Fig. 1c; S1) and the particles had 2 ± 0.14 of Calcium to Phosphorous (Ca:P) ratio (Fig. 1c).
F16BP microparticles are phagocytosed by DCs
To test if F16BP MPs could release F16BP, release kinetics in phosphate-buffered saline was performed. It was observed that F16BP MPs could release F16BP for 6 h in a sustained manner (Fig. S2). These data demonstrated that the MPs generated contain F16BP, are in the phagocytosable range of DCs and can release F16BP to potentially allow glycolysis to move forward. In addition to F16BP, particles of other control metabolites such as ribulose 5 phosphate (R5P), Phosphoenolpyruvic acid (PEP), and fructose-6 phosphate (F6P) were also generated (Fig. S3). In this study, PBS was chosen to mimic in vivo physiological conditions of phosphates, and the release of F16BP in this medium was determined22. Moreover, since activated DCs are expected to survive for 1–3 days in vivo upon phagocytosis, the short-term activity or stability of the F16BP MPs is desirable23.
To test if these particles can be phagocytosed by DCs, confocal imaging was performed. Specifically, F16BP MPs were generated with FITC intercalated within the particles. Bone marrow-derived DCs (DCs) were then incubated with these particles for 60 min, and stained for actin and nuclei, and fluorescent imaging was performed. Cytochalasin D in the presence of F16BP-FITC MPs were used as control. It was observed that DCs were able to associate with the particles effectively, and the confocal slices in the z-direction demonstrated that the particles were internalised (Fig. S4).
F16BP microparticles rescue glycolysis in DCs in vitro
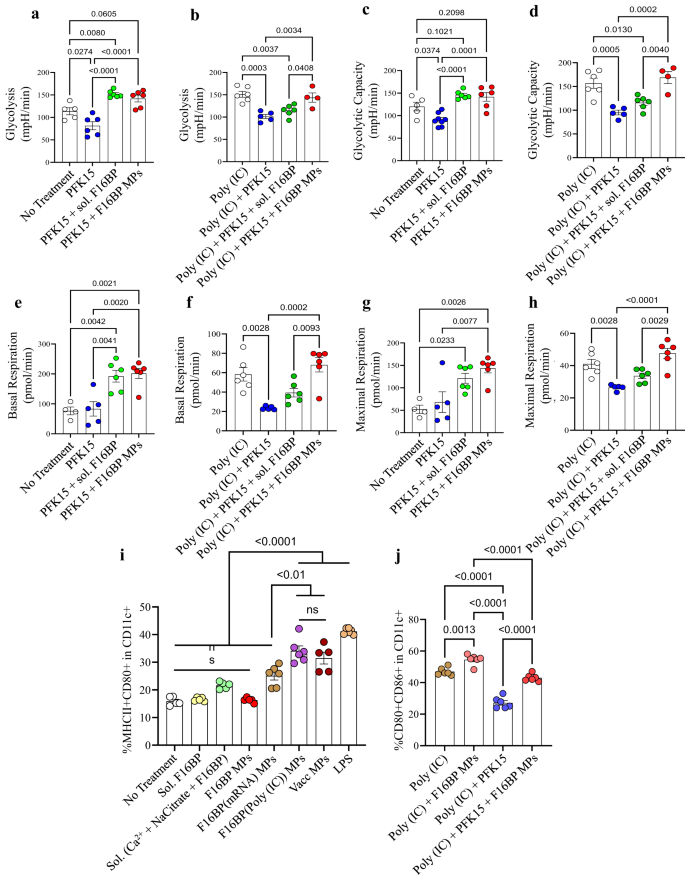
Next, the ability of the F16BP MPs to rescue glycolysis in DCs in the presence of glycolytic inhibitor PFK15 was tested using extracellular flux assays. DCs were cultured with either PFK15, F16BP MPs, soluble Fructose-6-Phosphate (F6P-upstream of PFKFB3) or PFK15 + F16BP MPs, or individual components of the F16BP MPs for 2 h, and extracellular acidification rate (ECAR–the rate of glycolysis) measurements were obtained. It was observed that PFK15 brought the glycolysis and glycolytic capacity (y-axis-ECAR) lower than the no-treatment control (Fig. 2a–d; S5). Importantly, glycolysis and glycolytic capacity were significantly higher in F16BP MPs even in the presence of the PFK15, as compared to the PFK15 alone control (Fig. 2a–d). Also, in the presence of poly(IC) (an activating agent for DCs)24, PFK15 decreased the glycolysis and the glycolytic capacity, and the F16BP MPs were able to rescue this decrease even in the presence of PFK15 (Fig. 2e–h). Overall, these data suggest that the phagocytosable F16BP MPs were able to rescue glycolysis in DCs even in the presence of glycolytic inhibitor PFK15, and thus can be utilised for immunotherapies where the glycolysis pathway of cancer cells is targeted. This is important since under conditions of inflammation, DCs need to perform enhanced levels of glycolysis to support the inflammatory protein production11,25.
a–d DCs treated with F16BP MPs rescued glycolysis and glycolytic capacity from glycolytic inhibition (PFK15), in vitro (n = 6; One-way ANOVA Tukey’s test), e–h DCs treated with F16BP MPs accelerate basal and maximal respiration even under glycolytic inhibition (PFK15), in vitro (n = 6; One-way ANOVA Tukey’s test). i Vaccine particles induced significantly higher frequency of MHCII + CD86+ in CD11c+ DCs as compared to the individual component controls of the MPs (n = 6; One-way ANOVA Tukey’s test). j F16BP MPs were able to rescue the activation of DCs even in the presence of PFK15 (PFK15 conc. = 25 µM) (n = 6; One-way ANOVA Tukey’s test). Data represented as mean ± std error.
F16BP microparticles rescue activation of DCs in vitro
To test if F16BP MPs can modulate the function of DCs, mRNA-based and peptide-based vaccine F16BP MPs were formulated. These vaccines were generated by intercalating di-phosphorylated melanoma peptide antigen Tyrosine-related protein 2 (pTRP2), and poly(IC) and mRNA derived from melanoma cells, which contain several phosphate groups in their backbone (Fig. S6). Since pTRP2p, and poly(IC) have phosphate groups, these molecules can be incorporated into the F16BP MPs using the calcium-phosphate chemistry. The amount of pTRp2 and poly(IC) incorporated within the F16BP MPs was determined to be 78 ± 3.4, and 50.9 ± 7.9 µg, respectively. Next, F16BP MPs intercalated with poly(IC) and pTRP2 were incubated with DCs overnight, and flow cytometry was utilised to test if these particles could activate DCs (Fig. 2i, j). It was observed that the vaccine particles induced significantly higher frequency of MHCII + CD86+ in CD11c+ DCs as compared to the individual component controls of the MPs (Fig. 2i). Additionally, it was observed that PFK15 was able to decrease the activation of DCs (CD80 + CD86+ in CD11c + ) even in the presence of poly(IC), and F16BP MPs were able to rescue the activation of DCs even in the presence of PFK15 (Fig. 2j). Also, calcium ions at the concentration that were added to the DCs as the F16BP MPs, did not lead to changes in activation (MHCII + CD80+ in CD11c + ) profile of DCs (Fig. S5). These data indicate that the F16BP-based vaccine MPs rescue DC activation even in the presence of PFK15, which is important if in vivo cancer vaccine responses need to be generated in the presence of glycolysis inhibition.
To further analyse if DCs treated with F16BP MP formulations modulate T-cell responses, a syngeneic mixed lymphocyte reaction (MLR) was performed. C57BL/6j bone marrow-derived DCs were treated with different conditions (Fig. S7) for 2 h and then cultured with T cells isolated from C57BL/6j mice for 60 h. The cells were then stained against CD4, CD8, CD44, Tbet, RORɣT, GATA3, CD25 and Foxp3, and analysed using flow cytometry. It was observed that the F16BP MPs, F16BP(pTRP2), F16BP(poly(IC), F16BP(pTRP2+poly(IC)), PFK15 + F16BP MPs, and PFK15 + F16BP(pTRP2+poly(IC)) all significantly upregulated the frequency of activated Th1, activated Th17, and activated Tc1 cells, while simultaneously downregulating the frequency of Th2, Tregs, and activated Th2 (Fig. S7). Interestingly, it was observed that the treatment of DCs with F16BP MPs led to the biggest changes in T-cell polarisation and activation. This change observed was even in the presence of adjuvant poly(IC) or the antigen pTRP2. Moreover, soluble F16BP and its components added to the DCs induced a significantly lower frequency of activated Th1, Tc1, and Th17 as compared to F16BP MPs in all possible combinations. These data suggest that the presence of particles was important for skewing pro-inflammatory T-cell frequencies in an MLR reaction.
F16BP vaccines with glycolytic inhibitors generate robust anti-tumour responses
To test if the immunometabolism modulating approach of rescuing glycolysis in the presence of glycolytic inhibitors and generating cancer vaccine immunotherapies, highly aggressive forms of melanoma mouse models were chosen. Specifically, vaccine MPs were injected subcutaneously in mice containing YUMM1.1 (murine BRAFv600e mutation similar to humans) melanoma tumours, and their ability to reduce tumour growth and modulate innate and adaptive immune responses was tested.
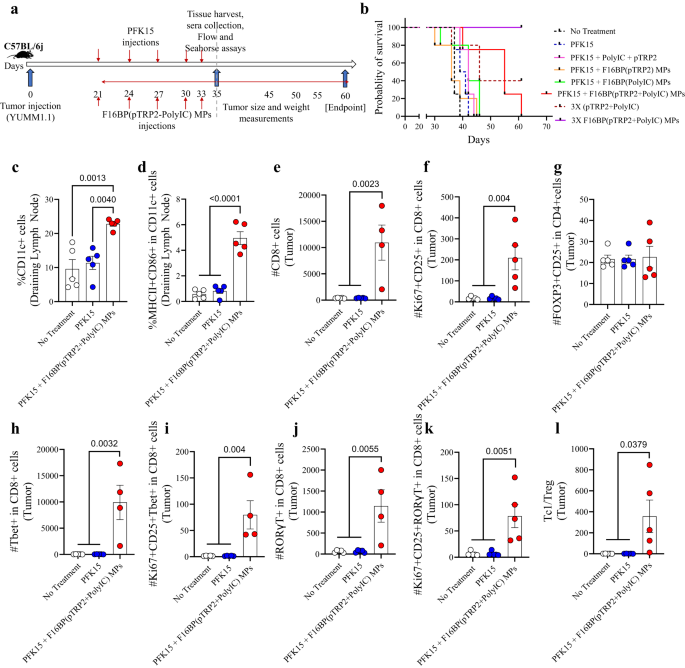
In this melanoma model, 0.75 × 106 YUMM1.1 cells were injected subcutaneously in C57BL/6j immunocompetent mice, and PFK15 was injected every alternate day for the duration of the study. Moreover, F16BP(pTRP2+poly(IC)) were injected subcutaneously on the same days as the PFK15 injections (Fig. 3a). In vitro it was determined that PFK15 was effective in preventing the proliferation of YUMM1.1 cancer cells (Fig. S8). It was observed that the treatment group of PFK15 + vaccine MPs led to significantly increased survival of mice (day 60 – endpoint) and slower tumour growth as compared to all the different controls (Fig. 3b; S9). This increase in survival in the vaccine MPs was associated with an increase in the DC population and activated DC population in the draining inguinal lymph nodes on day 35, which suggests that the vaccine MPs were able to modulate the innate immune responses (Fig. 3c, d). F16BP was essential in generating anti-tumour responses in mice since without F16BP mice did not survive beyond day 45. Moreover, 3 times the dosage of F16BP (pTPR2+ poly IC) MPs without PFK15 was able to abrogate existing tumours, which were not detected till day 60 (Fig. 3b; S9). Additionally, it was also found that the poly(IC) and pTRP2 needed to be incorporated within the F16BP MPs, and the injections of F16BP MPs with soluble (poly(IC) + pTRP2), did not reduce tumour growth in mice (Fig. S9). F16BP MPs by themselves might accelerate the glycolysis in different cells in mice, as was observed in vitro in DCs. This acceleration of glycolysis then might cause the immune response to be skewed toward anti-tumour responses. However, this control was not tested in mice and is a limitation of this study. Within the tumour, no significant differences were observed in the number of CD4 + T cells of different treatment groups; however, there was a significant increase in the proliferating and activated CD4+ cells in mice treated with vaccine MPs as compared to the other treatment groups (Fig. S10a). Furthermore, no significant differences were observed in the number of T-helper type 1 (Th1) and activated and proliferating Th1 cells; a significant increase in T-helper type 17 (Th17) and activated and proliferating Th17 cells were observed in mice treated with vaccine MPs as compared to other treatment groups (Fig. S10a). It was also observed that when mice were treated with PFK15 and with soluble pTRP2 and soluble poly (IC) without F16BP, it led to increased levels of activated DCs, as compared to a no-treatment control, however, DCs were not modulated in other organs (Fig. S10b). Furthermore, without F16BP, the formulation did not modulate pro-inflammatory T-cell responses as compared to the no-treatment control (Fig. S10c). These data suggest that F16BP was required in the formulation to generate pro-inflammatory T-cell responses. Moreover, within the tumour, there was a significant increase in the number of CD8 + T cells and proliferating and activated CD8 + T cells in mice treated with vaccine MPs as compared to other treatment groups indicating the decrease in tumour growth kinetics in mice treated with vaccine MPs responses (Fig. 3e, f). No significant differences were observed in regulatory T cells in the different treatment groups (Fig. 3g). Additionally, there was a significant increase in the number of Tc1, Tc17 and proliferating and activated Tc1 and Tc17 cells in mice treated with vaccine MPs as compared to other treatment groups (Fig. 3h–k). Also, a significant increase in the Tc1/Treg ratio was observed in mice treated with vaccine MPs as compared to other treatment groups (Fig. 3l). These data demonstrate that the vaccine MPs were able to generate robust pro-inflammatory adaptive immune responses in the tumour.
a Schematic representation of subcutaneous injection of Vacc MPs, in vivo, b Kaplan–Meir curve demonstrating significantly higher survival of mice treated with Vacc MPs, c, d Mice treated with Vacc MPs had a significantly higher percentage of the total as well as activated DCs in the draining lymph (n = 5; One-way ANOVA Tukey’s test), e, f Mice treated with Vacc MPs had significantly higher number of Tc and activated and proliferating Tc as compared to other treatment groups (n = 4 or 5; One-way ANOVA Tukey’s test), g No significant differences in the number of Tregs was observed across treatment groups (n = 5; One-way ANOVA Tukey’s test), h–k Mice treated with Vacc MPs had significantly higher number of Tc, Tc1, activated and proliferating Tc1 and Tc17 cells (n = 4; One-way ANOVA Tukey’s test), l Significantly higher Tc1/Treg ratio was observed in mice treated with Vacc MPs as compared to the control groups (n = 4; One-way ANOVA Tukey’s test). Data represented as mean ± std error.
F16BP vaccines maintain DC and T-cell metabolic function in vivo
To test if the DCs or T cells maintain their metabolic function after treatment, mice with tumours were euthanized and the cells from tumours, spleen and inguinal LNs were isolated. These cells were then cultured with 2NBDG and flow was utilised to determine the uptake of 2NBDG representing the level of glycolysis. It was observed that in the tumour, gMFI of 2NBDG in CD80+ DCs and macrophages were significantly higher in PFK15 + F16BP(polyIC) MPs as compared to PFK15 only condition, however, these were not significantly different than no-treatment control (Fig. S11a–g). In the spleen, the gMFI of 2NBDG in DCs, CD80+ DCs, but not in CD206+ DCs were significantly higher in PFK15 + F16BP(polyIC) MPs treated mice as compared to all the controls (Fig. S11h–j). These trends were reversed in macrophages isolated from the spleen of PFK15 + F16BP(polyIC) MPs treated mice as compared to all the controls (Fig. S11k–m). These data suggest that systemically the MPs differentially modulated glycolysis of DCs and macrophages. In the inguinal LNs, gMFI of 2NBDG also was upregulated in activated CD80+ DCs, and CD206+ DCs (Fig. S11n–s). The 2NBDG assay thus demonstrated that the DC and macrophage glycolysis was still maintained in a tumour, spleen and in draining inguinal LNs. A similar study was performed for adaptive T cells to understand glycolytic plasticity in these cells. Notably, it was observed that CD45- cells isolated from the tumour had significantly lower 2NBDG gMFI in PFK15, PFK15+ soluble F16BP + soluble poly(IC), and PFK15 + F16BP(polyIC) MPs treated mice as compared to the control of no-treatment (Fig. S12a). This data suggest that the MPs or soluble parts of the MPs were not able to substantially modulate the glycolysis of non-immune cells or these cells have a higher level of metabolic plasticity as compared to immune cells. Moreover, gMFI of 2NBDG in T-helper cells in the tumour was not significantly different in PFK15 + F16BP(polyIC) MPs as compared to the no-treatment control, and these two conditions were significantly higher than the other controls (Fig. S12b). The gMFI of 2NBDG in CD8 + T cells in the tumour was not significantly different in PFK15 + F16BP(polyIC) MPs as compared to the no-treatment control (Fig. S12c), but was higher than PFK15+ soluble F16BP + soluble poly(IC) condition. In the spleen, there were no significant differences observed in CD4 + T cells, however, CD8 + T cells had higher 2NBDG gMFI as compared to the controls in PFK15 + F16BP(polyIC) MPs treated mice, but not different than no-treatment control (Fig. S12d, e). Also, in the inguinal lymph nodes, PFK15 + F16BP(polyIC) MPs treated mice had lowered 2NBDG gMFI as compared to the no-treatment control (Fig. S12f, g). The T-cell 2NBDG assay suggests that in the tumour both CD4+ and CD8+ T cells maintain their glycolysis even after ex vivo culture, and thus may support anti-tumour responses in vivo. Overall, these data demonstrated that the vaccine MPs that deliver F16BP and can rescue DCs were able to generate robust immune responses against an aggressive form of melanoma tumours.
F16BP rescues adoptively transferred DC metabolism in mice
In addition to the subcutaneous vaccine strategy, adoptive transfer of DCs has been tested in clinics for the treatment of prostate cancer26,27,28. However, these strategies have not been very successful in clinics in part due to their low efficacy in survival improvement. To test the versatility of the F16BP-PFK15 system, another aggressive B16F10 melanoma model was chosen and the ability of adoptive transfer of DCs were loaded with the MPs was utilised as a treatment modality (Fig. 4a). In this model, DCs were loaded with F16BP MPs intercalated with mRNA isolated from B16F10 cancer cells, and poly(IC) (Vacc DCs) and adoptively transferred in mice containing B16F10 tumours. This cellular therapy’s ability to modulate adaptive immune responses, reduce tumour growth and improve survival was measured.
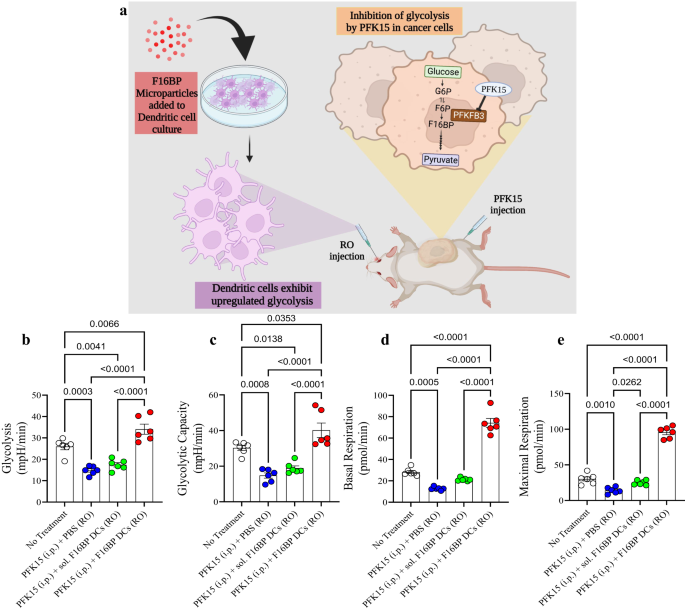
a Schematic representation of the adoptive cellular therapy model employed. b, c Mice injected with adoptively transferred DCs along with F16BP MPs were able to rescue glycolysis and glycolytic capacity from glycolytic inhibition (PFK15), in vivo (n = 6; One-way ANOVA Tukey’s test), d, e Mice injected with adoptively transferred DCs along with F16BP MPs accelerated basal and maximal respiration even under glycolytic inhibition (PFK15), in vivo (n = 6; One-way ANOVA Tukey’s test). Data represented as mean ± std error.
First, to test if the F16BP MPs (without poly(IC) or mRNA) can rescue DCs in vivo, F16BP MPs were loaded into DCs by incubating these particles with DCs for 2 h. Next, these DCs were adoptively transferred intravenously in mice and PFK15 was injected intraperitoneally. DCs were isolated from the spleen and extracellular flux assays were performed on these cells. It is expected that these DCs isolated from the spleen will be a mixture of both endogenous splenic DCs and adoptively transferred DCs. It was observed that the F16BP MPs were able to rescue glycolysis and glycolytic capacity of DCs as observed by the increased ECAR values in the presence of PFK15 as compared to the controls (Fig. 4b, c). Moreover, F16BP MPs were also able to rescue basal and maximal respiration of DCs in the presence of PFK15, as compared to the controls (Fig. 4d, e). This data demonstrated that these particles can not only rescue glycolysis and mitochondrial respiration in these cells in vitro but also in vivo, and thus might be able to generate functional immunotherapeutic responses.
Metabolic rescue of DCs generates robust adoptive cell immunotherapy
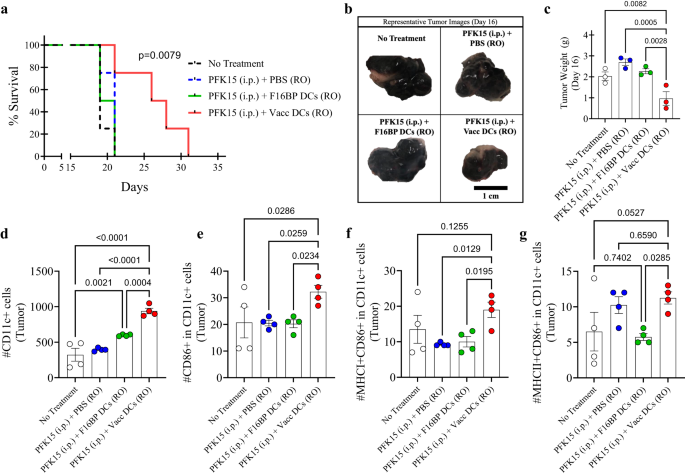
To test if the metabolic rescue in DCs can be applied to adoptive cell therapy, the adoptive transfer of DC vaccines was tested in mice (Fig. S13). First, in vitro, it was determined that PFK15 was effective in preventing the proliferation of B16F10 cancer cells (Fig. S14). Specifically, 0.75 × 106 B16F10 cells were injected in C57BL/6j immunocompetent mice, and PFK15 was injected every alternate day for the duration of the study (Fig. S13). Moreover, ex vivo DCs loaded with F16BP(mRNA+poly(IC)) (Vacc DCs) or DCs loaded with F16BP MPs were adoptively transferred using retro-orbital injections (RO) on days 6 and 19 (Fig. S13). It was observed that the survival of mice receiving Vacc DCs + PFK15 increased dramatically as compared to all the controls, and the tumour grew slower in mice receiving Vacc DCs as compared to the controls (Fig. 5a; S15). This data was further corroborated by tumour weights and images that were obtained at the half-point of the study on day 16 (Fig. 5b, c). These data indicate that after just two injections of Vacc DCs, there was a robust response against the tumour, which then reduced the tumour growth in mice.
a Kaplan–Meir curve demonstrating significantly higher survival of mice treated with adoptively transferred Vacc MPs (n = 10, p < 0.001), b Representative tumour images of different treatment groups on day 16. c Mice treated with adoptively transferred Vacc DCs had significantly lower tumour weights as compared to other treatment groups, in vivo (n = 3; One-way ANOVA Tukey’s test). d, e Significantly higher total, as well as activated DCs, were observed in mice treated with adoptively transferred Vacc DCs as compared to other treatment groups, in vivo (n = 4; One-way ANOVA Tukey’s test). f Significantly higher MHCI+ activated DCs were observed in mice treated with adoptively transferred Vacc DCs as compared to other treatment groups, in vivo (n = 4; One-way ANOVA Tukey’s test). g Significantly higher MHCII+ activated DCs were observed in mice treated with adoptively transferred Vacc DCs as compared to F16BP DCs, in vivo (n = 4; One-way ANOVA Tukey’s test). Data represented as mean ± std error.
Next, to test if the Vacc DCs could modulate the immune responses after their administration, on day 16, the spleen, lymph nodes, and tumours were isolated from different conditions and stained for the activation profile of DCs, and T cells. It was observed that the total number of DCs and activated DCs in tumours for Vacc DCs was significantly higher as compared to all the controls (Fig. 5d, e). Moreover, Vacc DCs also upregulated number of DCs that were MHCI + CD86 + CD11c+ in the tumour as compared to other treatment groups, suggesting the Vacc DCs support the activation of DCs in vivo (Fig. 5f). Also, Vacc DCs also upregulated number of DCs that were MHCII + CD86 + CD11c+ in the tumour as compared to adoptively transferred F16BP DCs, suggesting the Vacc DCs skew toward MHCII associated responses in vivo (Fig. 5g). These data indicate that glycolysis might be needed for potential infiltration of the DCs in the spleen and the tumour, and F16BP MPs provide the ability to DCs for glycolysis to occur.
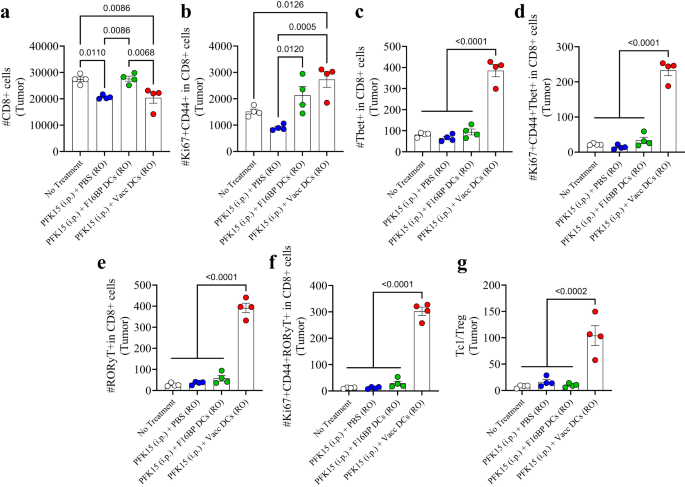
The adaptive T-cell responses in the tumour were skewed toward pro-inflammatory responses (Fig. 6a–g; S16). It was observed that PFK15 treatment alone significantly decreased the total number of CD8+ T cells in the tumour, and that DCs loaded with F16BP, were able to bring this number equal to the no-treatment control (Fig. 6a). Within this CD8+ T-cell numbers, it was observed that, there was 2–3-fold increase in activated and proliferating (Ki67+CD44+ in CD8+) T cells in the tumour in Vacc DC group as compared to the control groups (Fig. 6b). Furthermore, there was 4-fold significant increase in cytotoxic Tbet+ in CD8+ T cells (Tc1) in the Vacc DCs group as compared to all the other groups (Fig. 6c). Moreover, the number of activated and proliferating Tc1 cells was >10-fold higher in Vacc DCs group as compared to all the other treatment conditions (Fig. 6d). Similarly, the number of Tc17 cells (RORγt+ in CD8+), and activated and proliferating Tc17 (RORγt+Ki67+CD44+ in CD8+), which are emerging as an important pro-inflammatory cell types that induce cancer cell death, were also >10-fold significantly higher than the controls in the tumours (Fig. 6e, f). In addition to the Tc populations, Vacc DCs also led to a decrease in the numbers of T-helper cells (Th), activated and proliferating Th cells, Th1 (Tbet+ in CD4+), Th2 (GATA3+ in CD4+), activated and proliferating Th1 (Tbet+CD44+Ki67+ in CD4+), and activated and proliferating Th2 cells (GATA3+CD44+Ki67+ in CD4+) numbers in the Vacc DC group (Fig. S16). There was a significant decrease in the number of Treg (CD25+Foxp3+ in CD4+) within the tumour in mice treated with Vacc DCs as compared to untreated mice (Fig. S16). Moreover, there was a significant increase in the Th17 (RORγt+ in CD4 + ) and activated and proliferating Th17 cells (RORγt+Ki67+CD44+ in CD4+) within the tumour in the Vacc DC-treated mice as compared to all the other groups (Fig. S16). Furthermore, the ratio of Tc1 to Treg cells was 5–10-fold higher in the tumour of the Vacc DC-treated mice as compared to the controls (Fig. 6g). These data suggest that the Vacc DCs were able to induce a robust adaptive immune response against the tumours, which might be primarily driven by increases in Tc1 and Tc17 cell populations.
a, b Significantly modulation of total (CD8+) as well as activated and proliferating (Ki67+CD44+ in CD8+) cytotoxic T cells were observed in mice treated with adoptively transferred Vacc DCs as compared to other treatment groups, in vivo (n = 4; One-way ANOVA Tukey’s test). c–f Significantly higher total Tc1 (Tbet+ in CD8+), activated and proliferating Tc1 (Tbet+Ki67+CD44+ in CD8+), total Tc17 (RORɣT+ in CD8+), activated and proliferating Tc17 (RORɣT+Ki67+CD44+ in CD8+) were observed in mice treated with adoptively transferred Vacc DCs as compared to other treatment groups, in vivo (n = 4; One-way ANOVA Tukey’s test). g Significantly higher ratio of cytotoxic to regulatory T cells (Tc1/Treg) was observed in mice treated with adoptively transferred Vacc DCs as compared to other treatment groups, in vivo (n = 4; One-way ANOVA Tukey’s test). Data represented as mean ± std error.
Since Vacc DCs are administered systemically, spleen and cervical lymph nodes were also analysed for the changes in innate and adaptive immune responses (Figs. S17–S20). Notably, the overall frequency of DCs (CD11c+) and activated DCs (MHCII+CD86+ in CD11c+, MHCI+CD86+ in CD11c+, CD86+ in CD11c+, MHCI+ in CD11c+, MHCII+ in CD11c+) were significantly higher in spleen as compared to the controls; however, this difference was not observed in the cervical lymph nodes (Figs. S17 and S18). These findings suggest that the adoptive transfer of DCs primarily generates immune responses by modulating the innate immune responses in the spleen. Contrary to the tumour, it was observed that in the spleen, there were significant decreases in the Th17 frequency in Vacc DC groups as compared to all the controls (Fig. S19), significant increases in the Th2 frequency as compared to all the controls (Fig. S19), and a significant decrease in Tc17 frequency as compared to PFK15 alone, and PFK15 + F16BP DCs controls (Fig. S20). These findings indicate that although the Vacc DCs were administered systemically, the pro-inflammatory adaptive immune responses were primarily found in the tumour and not in the spleen. Additionally, the Vacc DCs isolated from the spleen of mice also maintained higher ECAR and OCR as compared to the controls (Fig. S21). To test if the F16BP component of the formulation is important for generating innate and adaptive immune responses DCs loaded with soluble mRNA + soluble poly (IC) were injected in mice retro-orbitally and the innate and adaptive immune responses generated in iLN, spleen and tumours were tested and compared to no-treatment control. It was observed that for increasing pro-inflammatory both DCs and T-cell responses F16BP loaded in DCs was essential, as there were no significant differences observed between no-treatment control and DCs loaded with soluble mRNA + soluble poly (IC) in these organs (Fig. S22).
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Automotive / EVs, Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- ChartPrime. Elevate your Trading Game with ChartPrime. Access Here.
- BlockOffsets. Modernizing Environmental Offset Ownership. Access Here.
- Source: https://www.nature.com/articles/s41467-023-41016-z