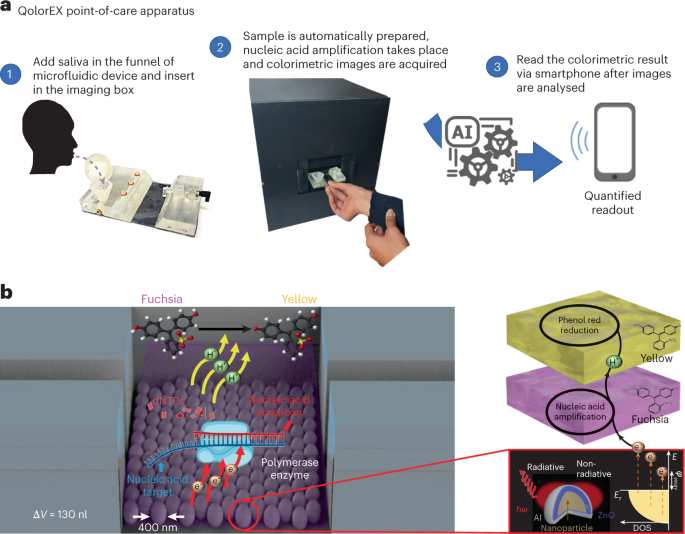
We propose a technique — QolorEX — for obtaining a label-free quantitative colorimetric readout at single nucleotide resolution, which can obtain a result within minutes. This approach integrates phenol red-based reverse transcription loop-mediated isothermal amplification (RT-LAMP) and rolling circle amplification (RCA) colorimetric assays3 with miniaturized plasmonic nanosurface microfluidics to achieve plasmonic hot-spot catalysis4. To streamline the amplification reaction, we developed a microfluidic cartridge that uses tunable angle-dependent microfluidic actuation to integrate the full cycle of sample collection, lysis, addition of assay reagents, nucleic acid amplification, and detection (Fig. 1a). To reduce user-introduced errors, we developed an illumination-coupled imaging box with automated actuators to handle, heat, and image the microfluidic cartridge without requiring input from the user. This automation allows the user to tap a button on a mobile phone application to begin the sequential operation of the microfluidic cartridge. The colorimetric readout is automatically detected by the complementary metal-oxide-semiconductor (CMOS) camera integrated with the imaging box. A machine-learning algorithm then analyses the readout and establishes positive or negative results; the result is then sent to the user’s mobile phone.
a, A schematic of the operation of QolorEX. The user spits saliva into the collection funnel and puts the microfluidic cartridge inside the imaging box. The results are then automatically relayed through a smartphone app. b, Upon light excitation, the plasmonic nanomaterial accelerates the amplification assay owing to the excess electrons at the reaction interface. The increased amplification rate increases the rate of production of protons, which decrease the pH of the medium, causing the phenol red to rapidly change colour from fuchsia to yellow in the presence of the target pathogen (shown in the inset). dNTP, deoxyribonucleotide triphosphate; DOS, density of states; E, energy; EF, Fermi energy; ϕassay, oxidation reaction initiation energy; ħω, photon energy; ΔV, sensing chamber volume. © 2023, AbdElFatah, T. et al.
We demonstrated that the rapid colorimetric readout obtained with QolorEX strongly depends on the injection of light-excited ‘hot’ electrons from the surface of self-assembled plasmonic nanoparticles into the mixture of sample and assay reagents in the sensing chamber. These hot electrons accelerate the nucleophilic reaction in the polymerization step of the amplification, which in turn results in the label-free pH-dependent colour transformation of phenol red from fuchsia to yellow (Fig. 1b). We found that plasmonic surfaces with 400 nm diameter nanoparticles offered higher electromagnetic-field enhancement and associated plasmonic hot-spot catalysis effects than plasmonic surfaces with other nanoparticle sizes, resulting in a 9-fold acceleration in the amplification reaction rate on average.
We validated the QolorEX system using various respiratory viruses and bacteria. We also demonstrated that QolorEX can differentiate between SARS-CoV-2 (sub)variants at the level of single nucleotide polymorphism. Additionally, QolorEX has a detection limit of 5 RNA copies per microlitre, which enables an accurate diagnosis from the onset of infection when viral loads are low. We also tested 33 SARS-CoV-2 positive and 15 negative human saliva samples. For these samples, QolorEX achieved a sample-to-answer time of 13 minutes with 95% accuracy, which is comparable to the gold standard quantitative PCR test. The limited user involvement, high accuracy, sensitivity, automation, and rapidity of QolorEX mean that this technique could address the need for PCR-grade tests in remote settings.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoAiStream. Web3 Data Intelligence. Knowledge Amplified. Access Here.
- Minting the Future w Adryenn Ashley. Access Here.
- Buy and Sell Shares in PRE-IPO Companies with PREIPO®. Access Here.
- Source: https://www.nature.com/articles/s41565-023-01398-z