| Mar 12, 2024 |
|
(Nanowerk News) Finding the best method to deliver chemotherapeutic drugs to tumor cells can be tricky. Ideally, the treatments target tumor cells while leaving healthy cells alone.
|
|
Immunoliposomes could be the answer. They can efficiently bind to antigens on tumor cell surfaces through their surface-targeting ligands, allowing tumor cells sufficient time to take up the “poison.” The benefits of immunoliposomes in cancer treatment have been extensively documented in the last four decades. However, immunoliposomal drugs have not yet made it to the market, although they had been demonstrated in laboratories since 1981.
|
|
Why? One key barrier is the lack of a large-scale, low-cost yet feasible manufacturing technique. Grafting targeting ligands onto plain liposomes to form immunoliposomes involves about a half-dozen steps and can lead to potential problems.
|
|
Yuan Wan, an associate professor at Binghamton University’s Thomas J. Watson College of Engineering and Applied Science, recently published research in the journal Nature Nanotechnology (“Chimeric nanobody-decorated liposomes by self-assembly”) outlining a one-step manufacturing process for immunoliposome manufacturing. It does not require any chemical conjugation and relevant chemical reagents, making it eco-friendly.
|
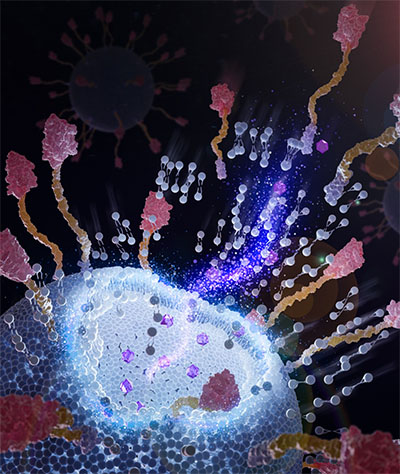 |
| Chimeric nanobodies on the surface of immunoliposomes can attach to tumor cells and facilitate better drug delivery. (Image: Courtesy of the researchers)
|
|
“The traditional manufacturing process of immunoliposomes is relatively complex,” said Wan, a faculty member in the Department of Biomedical Engineering. “It involves a lot of chemical conjugation and purification. Chemical conjugation and required reagents impair the stability and antigen binding of targeting ligands. The multistep process leads to payload leakage and product loss. So, immunoliposomes are less attractive to industrial manufacturers because of their low yield, high production costs and the high risk of batch-to-batch variation. These shortcomings hinder commercial production and clinical use of immunoliposomes.”
|
|
What makes Wan’s research different is the addition of engineered chimeric nanobodies, which have a “sticky” end. More than 2,500 nanobodies can integrate on the outside of a single 100-nanometer liposome, which is about 1,000 times smaller than a human hair.
|
|
This method is easier, faster and cheaper than traditional methods, and it allows for more control over the final product. The surface nanobodies also form a protective layer around the liposome, which might help it avoid being cleared by the body too quickly and allow it to stay in the bloodstream longer.
|
|
Another big advantage is that this method doesn’t require harsh chemicals. Traditional methods often use a substance called polyethylene glycol (PEG), which sometimes can cause problems for patients, even death. Because of these concerns, the federal Food and Drug Administration requires extra monitoring for drugs containing PEG.
|
|
“Something really interesting we found is that when these chimeric nanobodies are inserted into the lipid bilayer, they actually increase the rigidity and thermal stability of the entire immunoliposomes. So, the drugs packed inside can hold up for a good 10 months without obvious leaks,” Wan said.
|
|
Because there are also around 20 plain liposomal drugs already in use, Wan is hopeful that — with further research and medical trials —immunoliposomes could be manufactured and get federal approval for clinical use.
|
|
“We are also working on developing new chimeric nanobodies to ramp up production by at least 30 times. It will make the manufacturing cost of these chimeric nanobodies much lower.” Wan said.
|
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nanowerk.com/nanotechnology-news3/newsid=64839.php




