| Feb 27, 2024 |
|
(Nanowerk News) Zinc-air batteries are an inexpensive, powerful battery alternative that can be used on the small scale to power electronics or on the large scale for electric vehicles or energy storage. These batteries work when oxygen from the air oxidizes zinc, but the difficulty in oxygen activation which degrades battery performance has prevented their wide commercial adoption.
|
|
Information presented in a paper published in Carbon Future (“Fullerene-metalloporphyrin co-crystal as efficient ORR electrocatalyst precursor for Zn-air batteries”) shows how the addition of fullerene-derived carbon materials as catalysts can improve performance, stability, and cost of zinc-air batteries.
|
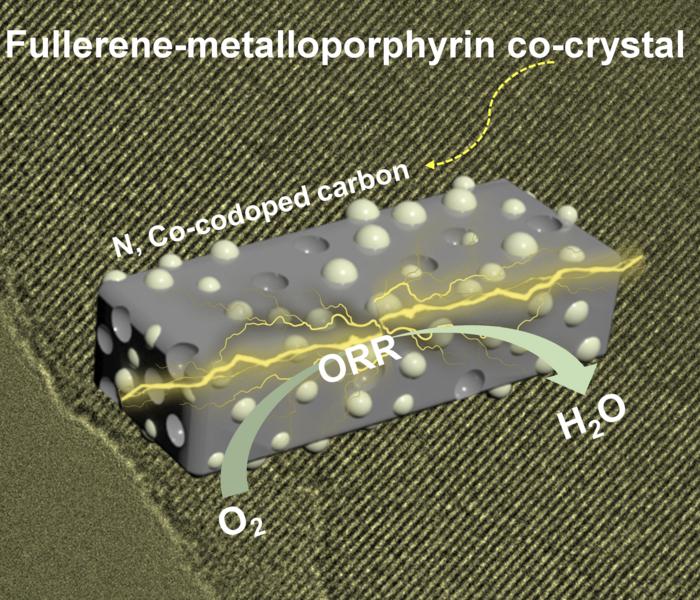 |
| This graphic illustrates a zinc-air battery can using a fullerene-metalloporphyrin co-crystal as an oxygen reduction reaction catalyst. (Image: Carbon Future, Tsinghua University Press)
|
|
“The sluggish kinetic characteristics caused by the difficulty in oxygen activation, oxygen to oxygen bond cleavage, and oxide removal of oxygen reduction in zinc-air batteries have limited their application in the commercial field,” said Fang-Fang Li, a professor at the School of Materials Science and Engineering at Huazhong University of Science and Technology in Wuhan, China. “Carbon-based non-noble metallic catalysts have been regarded as promising materials for oxygen reduction reaction due to their large surface areas, high electrical conductivity, outstanding mechanical properties, and excellent stability in electrochemical environments.”
|
|
Fullerene is an allotrope of carbon with a closed cage structure in the shape of a soccer ball. Pure fullerene has insufficient conductivity that limits electron transfer, but crystals derived from fullerene have an improved specific surface area, conductivity, and active sites. Fullerene crystals are created through a process called liquid-liquid interface precipitation. During this process, fullerene is dissolved in two different solvents and crystals are formed at the interface of the two liquids.
|
|
Researchers then created a supramolecule combining fullerene crystals with metalloporphyrin, a molecule with a unique structure. They created four versions of this supramolecule to try and optimize it to the best performance. Three were heated to different temperatures (700°C, 800°C, and 900°C) and then the final sample was also heated to 800°C, but mixed differently from the other samples without the liquid-liquid interface precipitation method.
|
|
Before testing the performance of the fullerene-metalloporphyrin supramolecule, researchers studied the structural features of the sample through scanning electron microscopy and X-ray diffraction, Raman spectroscopy, and additional measurements. They found that the liquid-liquid interface precipitation method increased defects, which improved the performance of the oxygen reduction reaction. They also consistently found that the supramolecule that was heated to 800°C performed better than the others that were tested over the course of the experiment and they moved forward with testing this supramolecule in a practical application.
|
|
To test the performance of the fullerene-metalloporphyrin supramolecule, researchers built a homemade zinc-air battery, using the fullerene-metalloporphyrin as the cathode. “The results highlight the exceptional long-term stability exhibited by fullerene-metalloporphyrin. The optimized zinc-air battery performance of fullerene-metalloporphyrin underscores the robust and enduring electrocatalytic performance of the supramolecule. This combination of high power density and extended stability positions fullerene-metalloporphyrin derived carbon material as a highly promising catalyst for practical applications of zinc-air batteries,” said Li.
|
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nanowerk.com/nanotechnology-news3/newsid=64739.php




