The last time the NMPA In Vitro Diagnostic Reagents Classification Catalog was announced was in 2013, with two supplemental updates in 2017 and 2020 respectively. NMPA issued the “Draft Classification Catalog for IVD Reagents” on March 14, 2023, to “keep up with the regulatory and industry development needs brought by new technologies, methods and biomarkers.”
The new Catalog is the result of several years in effort to standardize the classification and streamline the IVD registration process.
Feedbacks need to be submitted by April 20, 2023. When finalized, new registration application for IVD reagents should follow the new classifications.
Compared with the previous catalogs, key changes are:
- The number of IVDs catalogs increased to 25:
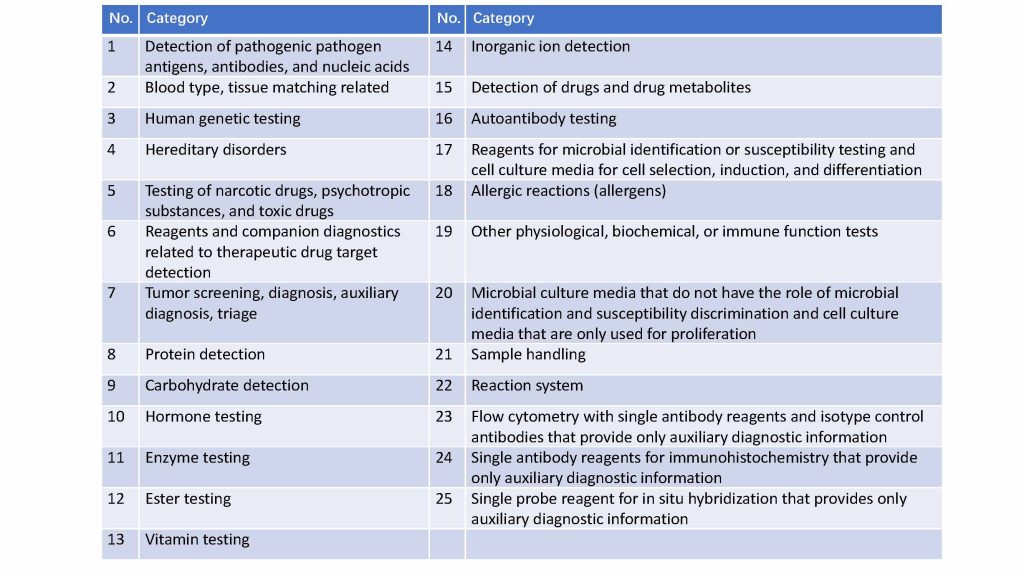
- There are 2046 subcategories, classified by biomarkers. Among them are 401 Class III and 759 Class II IVD reagents.
- The tumor markers for monitoring and prognosis observation are classified into Class II “Reagents for protein detection”.
- The medium for microbiological identification, such as the chromogenic identification medium with indicator, is classified as Class II; Microbial culture media for selective cultivation through the regulation of nutrients or antibiotics is classified as Class I.
- “Reagents for sample treatment”, classified as Class I, mainly refer to the general products used in the sample pretreatment stage before the detection reaction occurs, and does not participate in the reaction. Such products only include reagents for sample processing that are common to the instrument platform or methodology, and do not include reagents for sample processing that are specific to specific test items.
- “Reagents for reaction system”, classified as Class I, mainly refer to the general reagent used to maintain the reaction system environment in the detection reaction stage. It only includes reaction system reagents that are common to the instrument platform or methodology, such as substrate solution for chemiluminescent immunoassay, luminescent solution for chemiluminescent immunoassay, etc., not specific to specific test items.
For an English copy of the IVD Classification Catalog, please email info@ChinaMedDevice.com. We charge nominal fees for the translation.
For IVD Classification Rules released in October 2021, please click HERE
- SEO Powered Content & PR Distribution. Get Amplified Today.
- Platoblockchain. Web3 Metaverse Intelligence. Knowledge Amplified. Access Here.
- Source: https://chinameddevice.com/ivd-reagents-classification-catalog/



