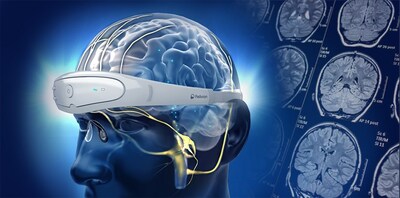
TAMPA, Fla., Jan. 9, 2024 /PRNewswire/ — Neurolief, a global leader in medical devices delivering groundbreaking neurotechnology innovations for the treatment of Neurological and Neuropsychiatric disorders, announces the approval of its neuromodulation device, Relivion®, by the Japanese Ministry of Health, Labor and Welfare (MHLW). This approval marks a historic moment for Neurolief and its partner Sawai Pharmaceutical Co., Ltd, as Relivion® becomes the first neuromodulation device approved in Japan for at-home use in the acute treatment of migraine.
Relivion® is a novel, non-invasive multi-channel brain neuromodulation technology. It stands out by concurrently stimulating the occipital and trigeminal nerve branches in the head, effectively modulating brain networks associated with debilitating migraine headaches. The system is complemented by a state-of-the-art patient mobile app and a physician interface featuring cloud-enabled data-tracking capabilities, allowing seamless AI integration and enabling remote patient monitoring.
Marketing efforts for Relivion® will be led by Sawai Pharmaceutical Co., Ltd, which have entered into an exclusive partnership agreement with Neurolief for development and commercialization of Relivion® for migraine and depression indications in Japan.
Scott Drees, CEO of Neurolief, expressed great enthusiasm for the MHLW approval, emphasizing the profound impact this decision will have on the millions of migraine patients in Japan. He underscored this achievement as a pivotal step in realizing Neurolief’s mission of bringing its pioneering neurotechnology to individuals globally.
Motohiko Kimura, President at Sawai pharmaceutical, emphasized the company’s unwavering dedication to prioritizing patients through Sawai’s corporate philosophy of ‘Always Putting Patients First’. This commitment reflects Sawai’s strong resolve to contribute to healthier lives. With the upcoming introduction of the Relivion® neuromodulation system in Japan, Sawai aims to elevate the landscape of acute migraine treatment options which is currently limited primarily to drug therapy.
About Neurolief
Neurolief is a leading brain neurotechnology company dedicated to enhancing the lives of patients suffering from Neurological and Neuropsychiatric disorders. Comprising experienced professionals with a proven track record in neuroscience, neuromodulation technology and the neurotech devices industry, the company has developed Relivion®MG for the treatment of migraine and Relivion®DP for Major Depressive Disorder (MDD). These are the world’s first and only non-invasive multi-channel brain neuromodulation technologies designed to concurrently stimulate the occipital and trigeminal nerve branches in the head, thereby modulating brain networks associated with migraine and depression. Relivion®MG is currently FDA, CE and MHLW approved for the acute treatment of migraine and aims to provide a highly effective alternative to pharmaceutical therapies. The system also includes a patient mobile app and a physicians’ interface with cloud-enabled data-tracking features, enabling AI incorporation and remote patient monitoring. The company is actively pursuing FDA and CE mark approvals for the treatment of Major Depressive Disorder (MDD).
For additional information on Neurolief and Relivion®, please visit our website at www.relivion.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/neuroliefs-relivion-secures-regulatory-approval-in-japan-pioneering-at-home-migraine-treatment-302029104.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/neuroliefs-relivion-secures-regulatory-approval-in-japan-pioneering-at-home-migraine-treatment-302029104.html
SOURCE Neurolief Inc.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.biospace.com/article/releases/neurolief-s-relivion-secures-regulatory-approval-in-japan-pioneering-at-home-migraine-treatment/?s=93