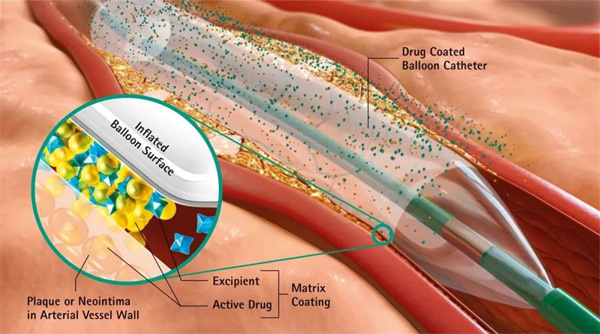
NMPA published the “Draft Guideline on Clinical Trial of Peripheral Drug-Coated Balloon Catheters” on February 5, 2024 for feedback. Feedback needs to be submitted by February 26, 2024.
This guideline proposed to provide a structured approach to the clinical evaluation of peripheral drug-coated balloon catheters, emphasizing rigorous study design, ethical considerations, and a focus on safety and efficacy endpoints. Manufacturers and applicants are encouraged to adhere to these guidelines to facilitate regulatory approval.
Application Scope
This guideline is applicable to routinely designed peripheral drug-coated balloon catheters, which is Class III devices, used in percutaneous transluminal angioplasty (PTA). It is for the treatment of peripheral arterial obstructive diseases, including diseases affecting the femoropopliteal artery, infrapopliteal artery, and stenotic lesions in peripheral autogenous arteriovenous fistula for hemodialysis access.
Highlights in the Guideline
Clinical Trial Design
- Trial Objectives:
- Clearly defined and specific trial objectives focusing on assessing clinical safety and efficacy.
- Emphasis on evaluating device effectiveness for meeting clinical needs.
- Trial Design Type:
- Recommendation for well-designed prospective, randomized, and controlled multicenter trials.
- Control group selection criteria: Similar devices with good clinical efficacy already marketed domestically.
- Comparator Selection:
- Selection of comparable devices for efficacy evaluation, with options for superiority, equivalence, or non-inferiority testing.
- Attention to threshold determination for ensuring clinical benefits outweigh risks.
- Inclusion and Exclusion Criteria:
- Definition of participant selection criteria based on factors like indications, disease classification, disease severity, anatomical location, and patient age.
- Outcome Assessment:
- Primary Endpoints:
- Evaluation of safety and efficacy with consideration for anatomical and clinical endpoints.
- Secondary Endpoints:
- Evaluation of various endpoints based on applicability, including limb patency, clinical-driven target vessel revascularization, and adverse events.
- Sample Size Estimation:
- Adequate sample size to ensure robust results, considering trial design, primary endpoints, and statistical requirements.
- Follow-up Duration:
- Definition of clinical follow-up times post-surgery, with a recommendation for continued monitoring up to two years for assessing long-term safety and efficacy.
Additional Considerations
- Applicability Support:
- Clinical evaluation data should support the declared product scope, including considerations for special cases such as long lesions.
- Subgroup Analysis:
- Predefined subgroups in clinical trial design for specific situations, with comparative analysis in trial reports.
- Procedural Clarifications:
- Explicit mention of routine pre-dilation operations and clarity on post-dilation, concurrent drug usage, and complementary treatment devices during the surgical process.
Utilizing Overseas Clinical Trial Data
- Conditions for Acceptance:
- Overseas clinical trial data for peripheral drug-coated balloon catheters can be referenced if it complies with local registration requirements, ethical, legal, and scientific principles, and is scientifically robust and complete.
- Guidance Reference:
- The “Technical Guidelines for Accepting Overseas Clinical Trial Data of Medical Devices” should be consulted for submitting clinical trial data.
The draft guideline has two annexes:
- Considerations for Clinical Trials of Peripheral Drug-Coated Balloon Catheters: Femoropopliteal Artery
- Considerations for Clinical Trials of Peripheral Drug-Coated Balloon Catheters: Peripheral Arteriovenous Fistula
For an English copy of the draft guideline, please email info@ChinaMedDevice.com.
For Registration Draft Guideline of Drug-Coated Balloon Dilatation Catheter, please click HERE
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://chinameddevice.com/peripheral-drug-coated-balloon-catheter/



