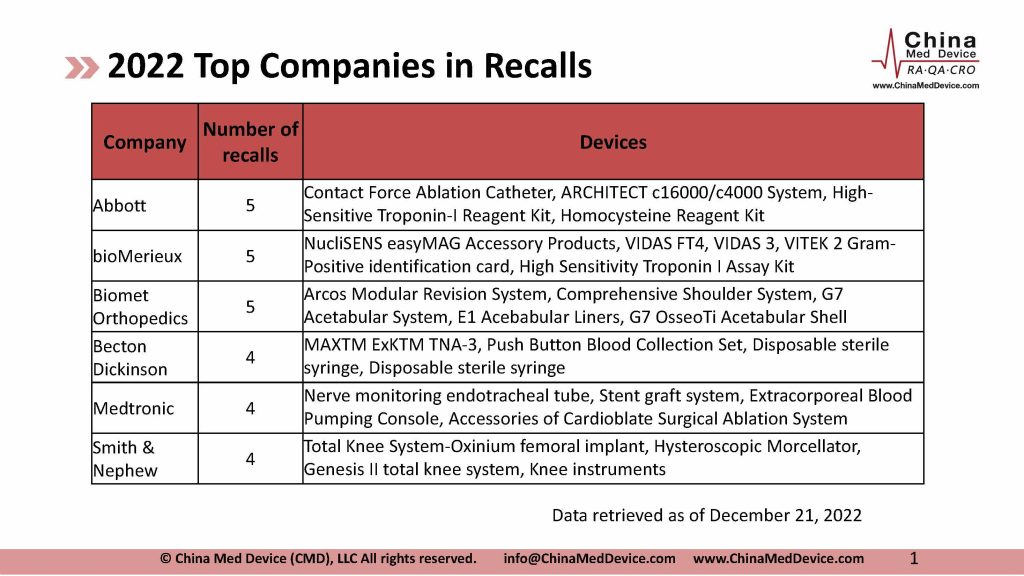
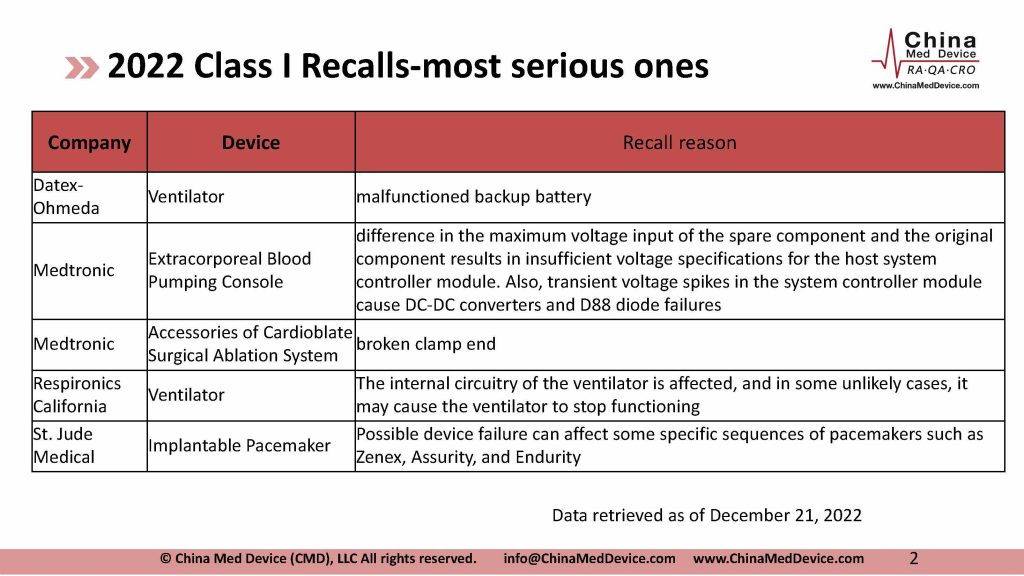
There have been 92 recalls from overseas manufacturers reported to NMPA in 2022, as of December 21. Many big names are on the list. Abbott, bioMerieux, Biomet, BD, Medtronic, and Smith & Nephew are among the most recalls.
China Med Device has summarized NMPA recall notices into an excel spreadsheet. Email info@ChinaMedDevoce.com to get an English copy.
Recall Regulation
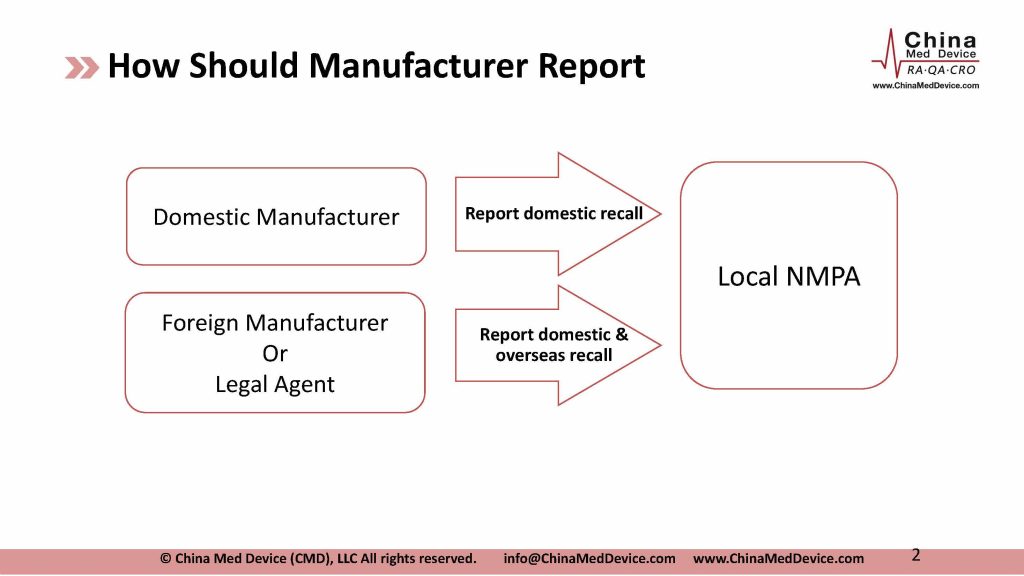
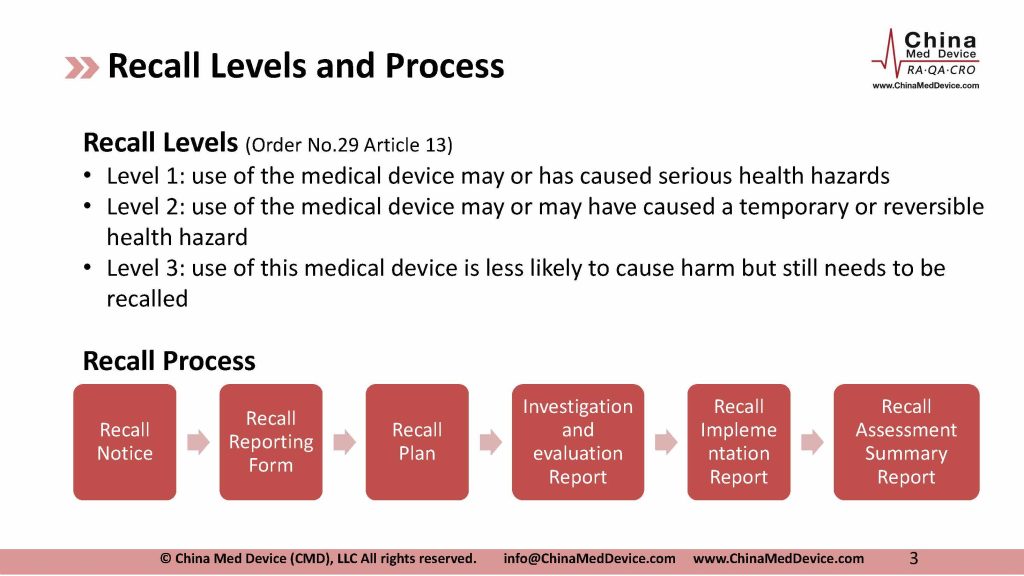
“Administrative Measures for the Recall of Medical Devices” released in February 2017 stipulates that “medical device manufacturers conduct recall defective products by warning, inspection, repair, re-labeling, modification, and improvement of IFU, software update, replacement, recovery, destruction, etc. Foreign manufacturer can designate an agent in China to conduct the recall.
The “defective” medical device products have the following scenarios:
- Products with unreasonable risks that may endanger human health/life under normal use
- Products that do not meet the technical requirements or mandatory standards
- Products that do not meet the relevant production and qualify management regulations which may lead to unreasonable risks
- Other products that need to be recalled
- SEO Powered Content & PR Distribution. Get Amplified Today.
- Platoblockchain. Web3 Metaverse Intelligence. Knowledge Amplified. Access Here.
- Source: https://chinameddevice.com/recalls-overseas-manufacturers-2022/



